Ultra-preconcentration and determination of organophosphorus pesticides in soil samples by a combination of ultrasound assisted leaching-solid phase extraction and low-density solvent based dispersive liquid–liquid microextraction†
Mehrnoush Mohammadia,
Hamed Tavakoli *b,
Yaser Abdollahzadehc,
Amir Khosravid,
Rezvan Torkamane and
Ashkan Mashayekhia
*b,
Yaser Abdollahzadehc,
Amir Khosravid,
Rezvan Torkamane and
Ashkan Mashayekhia
aDepartment of Chemical Engineering, Faculty of Engineering, South Tehran Branch, Islamic Azad University, P.O. Box 11365-4435, Tehran, Iran
bThe Young Research Club of the Islamic Azad University, Nour Branch, Nour, Iran. E-mail: ha.tavakoli159@yahoo.com
cArvin Bonyan Tajhiz Company, Tehran, Iran
dYoung Researchers and Elites Club, North Tehran Branch, Islamic Azad University, Tehran, Iran
eOil and Gas Centre of Excellence, School of Chemical Engineering, University College of Engineering, University of Tehran, Tehran, Iran
First published on 18th August 2015
Abstract
An ultra-preconcentration technique composed of ultrasound assisted leaching-solid phase extraction (USAL-SPE) and low-density solvent based dispersive liquid–liquid microextraction (LDS-DLLME) coupled with gas chromatography-mass spectrometry (GC-MS) was developed for preconcentration and determination of organophosphorus pesticides (OPPs) in soil samples. Parameters that affect the efficiency of the procedure were investigated by a fractional factorial design (FFD). Afterwards, variables showing significant effects on the analytical responses were considered using response surface methodology (RSM) based on a central composite design (CCD). Under optimum conditions, the enrichment factors were 7215–9842, the linear range was 0.012–625 ng g−1 and limits of detection (LODs) were between 0.002 and 0.125 ng g−1. The relative standard deviations (RSDs) were in the range of 5.4–8.3% (n = 6), and the relative recoveries of OPPs from different soil samples were 84–98%. The proposed methodology constitutes a suitable approach for the analysis of OPPs in complex soil samples and requires minimum organic solvents consumption, sample manipulation and increased sample throughput.
1. Introduction
Soil is an important component of the ecosystem and closely related to human survival. As a part of the human environment, contaminated soil may cause a serious risk to human health. Organophosphorus pesticides (OPPs) enter the soil ecosystem because of direct spraying on the soil surface during pesticide application in agriculture; the drop from the foliage and stems occurs due to washing by rain and the rotting of plant bodies containing OPPs residues in the soil.1 These compounds are characterized by their toxicity, relatively high volatility, as well as their capacity to interfere with cell biochemistry when accumulated in organic tissues in the human body. Pesticides may cause acute anemia, bone structure disorders, as well as teratogenic and embryologic diseases;2,3 therefore, the analysis of OPPs residues in the soil plays an important role in environmental protection and human health.Determination of OPPs is usually performed by sample preparation methods coupled with gas chromatography-mass spectrometry (GC-MS),4–6 gas chromatography-nitrogen phosphorus detector (GC-NPD),7,8 gas chromatography-flame photometric detector (GC-FPD),9,10 high performance liquid chromatography-mass spectrometry (HPLC-MS)11 and high performance liquid chromatography-photodiode array detector (HPLC-DAD).12,13 Before analysis, due to the complexity of soil sample matrices, their incompatibility with the desired instrumental method and low concentrations of the analytes in soil, a preliminary sample preconcentration and/or separation technique is required. For the determination of OPPs, the separation and preconcentration methods reported in the literature are usually based on techniques such as supercritical fluid extraction,2,14 microwave-assisted extraction,4,15 pressurized liquid extraction,16,17 ultrasonic extraction18 and solid phase extraction (SPE).19 Various configurations of microextraction techniques have frequently been reported as alternatives to the classical approaches in the literature. Solid phase microextraction20 and liquid phase microextraction7,21 are fairly new methods of sample preparation for the preconcentration of OPPs, and have been proved to be simple, inexpensive, fast and virtually solvent-free sample pretreatment techniques with extensive application. Room temperature lixiviation is another method for extracting organic compounds from soil samples. It can be assisted by auxiliary energies such as ultrasonic radiation in order to favor the kinetics of the mass-transfer process of the target analytes to the liquid phase. This leads to an increment in the extraction efficiency of the technique in a minimum amount of time.22,23
Dispersive liquid–liquid microextraction (DLLME) is an analytical technique recently developed by Rezaee and co-workers.24 To date, DLLME has been applied to the analysis of various organic and inorganic pollutants in aqueous samples.25–27 However, there is some inconvenience in retrieving the organic phase. Classical DLLME avoids this problem with the use of organic extraction solvents with densities higher than water such that, after extraction, the extractant can be sedimented by centrifugation. To broaden the applicability of DLLME, several recent studies have focused on the use of organic solvents with lower densities than water.28–32 Very recently, Cabuk and co-workers proposed a new technique for the collection of an extraction solvent lighter than water after the DLLME procedure.33 After extraction, a disposable glass Pasteur pipette and anhydrous Na2SO4 (acts as a flow stopper) were used to remove the upper organic extract.
The main disadvantage of the DLLME is that it is not a selective extraction technique and also fails if phases do not separate even after centrifugation (in the case of heavily contaminated extracts). Thus, in order to overcome this problem, it is necessary to include a clean-up stage prior to this technique. SPE is widely used as a sample clean-up and a preconcentration technique in sample preparations. Recently, SPE combined with dispersive liquid–liquid microextraction showed a potential ability to isolate the target analytes from the complex samples and reduce matrix effects.34,35 This method can provide a very high enrichment factor and high selectivity.36 The use of SPE after USAL would increase the extraction efficiency of the LDS-DLLME technique and extend its applicability to soil samples. To the best of our knowledge, there are no reports about the use of SPE-LDS-DLLME-GC-MS after ultrasound leaching for the analysis of OPPs in soil samples.
In this study, the USAL-SPE-LDS-DLLME technique is proposed for the extraction and isolation of OPPs from soil, while determination is achieved by GC-MS analysis. For this purpose, four OPPs commonly found in environmental samples were selected as target analytes: diazinon, chlorpyrifos, thionazin and o,o,o-triethyl phosphorothioate. The influence of several variables, such as the volume of extraction solvent and disperser solvent, extraction time, salt effect, flow rate of sample solution and volume of sample, was studied and optimized with the aid of response surface methodology and experimental design. A fractional factorial design (FFD) was used to screen the significant factors. Then, a central composite design (CCD) was used to conduct a second-order mathematical model relating the enrichment factor to significant independent variables. Optimum conditions were predicted by using the mathematical model and three-dimensional (3D) response surfaces obtained from it.
2. Experimental
2.1. Reagents
OPPs (diazinon, chlorpyrifos, thionazin, o,o,o-triethyl phosphorothioate) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 0.005 g of each analyte (OPPs) was dissolved in 5.0 mL HPLC grade methanol obtained from Caledon (Ontario, Canada) to prepare a standard solution of 1000 mg L−1. All solutions were stored at 4 °C protected from light. 1-Dodecanol, 1-octanol and toluene as extraction solvents, and ethanol, acetone and acetonitrile as disperser solvents were purchased from Merck (Darmstadt, Germany). All the other chemicals used were of reagent grade or of the highest purity available. Ultrapure water (18 MΩ cm resistivity) was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). Plastic and glassware used for the experiments were previously washed with acetone and rinsed carefully with double distilled water.2.2. Equipment and working conditions
GC-MS analyses were performed on an Agilent 7890A gas chromatograph (Palo Alto, CA, USA) interfaced to an Agilent 5975C mass selective detector (MSD). The GC-MS system was equipped with a split/splitless injector system and a RXI-5 MS fused silica capillary column with 30 m length, 0.25 mm internal diameter and 0.25 μm film thickness. Helium (purity 99.9999%) was used as the carrier gas at a flow rate of 1 mL min−1. The injection port temperature was 260 °C and was used in the splitless mode. The oven temperature program was set up as follows: start at 45 °C, hold for 2 min; increase at 10 °C min−1 to 160 °C; increase at 3 °C min−1 to 180 °C; and finally increase at 12 °C min−1 to 270 °C, hold for 10 min. The ion source, quadrupole, and transfer line temperatures were set to 250 °C, 230 °C, and 280 °C, respectively. The MS system was operated in the full-scan mode with a mass range from m/z 45 to 400. The chromatographic peak areas of analytes were identified by comparison with retention time and mass spectra of authentic standards. The analytes were analyzed in selective ion monitoring mode for quantitative determination. The monitored ions of the analytes were selected based on their good selectivity and high sensitivity and were set as follows: diazinon, m/z 179; chlorpyrifos, m/z 314; thionazin, m/z 107; o,o,o-triethyl phosphorothioate, m/z 198.A 40 kHz and 0.138 kW ultrasonic water bath (Tecno-Gaz SpA, Italy) was employed for assisting the ultrasound leaching process. A centrifuge (model Z200A, HERMLE, Germany) was used for centrifuging the mixtures. SPE of OPPs was performed by using 100 mg of C18 sorbent with a 3 mL syringe barrel (Waters, Massachusetts, USA).
2.3. Sampling and sample preparation
Soil samples were collected from cucumber and cabbage farms (Isfahan, Iran), as well as flat, citrus and greenhouse soils (Rudsar, Iran). The cucumber and cabbage farms were sprayed with the OPPs 5 and 8 days before sampling, respectively. The soil sample used in this study as blank was collected from an ecological agriculture farm, in which no organophosphorus pesticides had been used during the past three years. The samples were air dried, sieved to size less than 1 mm and kept in airtight amber glass containers at 2 to 4 °C.For the preparation of blank soil samples, 100 g of the ecological agriculture farm soil sample was immersed in 200 mL of methanol, acetone, dichloromethane and n-hexane consecutively and stored for at least 24 h.4,37 The treated soil sample was spread out on a tray and air-dried in a fume hood to remove as much solvent as possible. The dried soil sample was stored in an airtight amber glass container at 2 to 4 °C. The prepared blank soil sample was analyzed before spiking and no detectable levels of the target compounds were found.
The fresh spiked soil samples were prepared by weighing two grams of the prepared blank soil sample and spiking with an appropriate amount of OPPs standard solution. The fresh spiked soil samples were immediately used in the USAL-SPE-LDS-DLLME procedure after being air dried for solvent evaporation.
2.4. The procedure
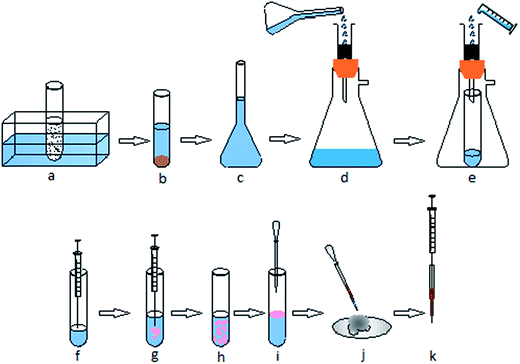
A schematic of the extraction procedure is shown in Fig. 1. 2 g of fresh spiked soil samples were placed into a 20 mL centrifuge tube, followed by the addition of 2.5 mL of methanol. The mixture was sonicated in a ultrasonication bath for 2 min. The resulting slurry was centrifuged at 5000 rpm (2906.8 × g rcf) for 5 min and the supernatant liquid was passed through a PTFE syringe filter (13 mm × 0.22 mm ) to remove particles.21 An aliquot of the residual filtrate was placed in a 50 mL cylinder and diluted with double distilled water to 50 mL. Then, the final test portion extract (50 mL) was loaded into a C18 SPE sorbent at a flow rate of 15 mL min−1 with the aid of a Rotavac vacuum pump (Heidolph, Germany). The C18 SPE cartridge was rinsed with 2 mL of double distilled water to remove matrix interferences. After ventilating of the solid phase, the desired compounds were eluted with 1 mL acetonitrile, which was used as the disperser solvent in the subsequent LDS-DLLME procedures. For LDS-DLLME, 10 μL of toluene was added to the SPE acetonitrile extract (1 mL). The resulting mixture was injected rapidly into 5 mL of double distilled water by using a syringe. A cloudy solution was formed due to the dispersion of tiny toluene droplets in the aqueous solution. The mixture was centrifuged for 2 min at 5000 rpm (2906.8 × g rcf). By this process, tiny toluene droplets floated on the surface of the aqueous solution due to their low density. The organic solvent together with some of the aqueous phase was pipetted by using a disposable glass Pasteur pipette. Next, the flow of the aqueous phase was stopped by successive dipping of the capillary tip of the pipette into anhydrous Na2SO4.33 The upper organic layer was then removed by using a 10 μL microsyringe and 1 μL of this solution was injected into the GC-MS for analysis.2.5. Study of experimental variables involved in USAL-SPE-LDS-DLLME
Different variables can affect the extraction yield in the USAL-SPE-LDS-DLLME procedure, and in most cases they are correlated. Therefore, optimization through a multivariate approach is recommended. However, some of them might not have a significant effect, and thus can be obviated. In this respect, a screening step, prior to the optimization step, is helpful in order to assess the significant variables involved in the analytical system under study. In this case, based on the literature,34–36 the influence of six variables, i.e., extraction solvent volume, disperser/eluting solvent volume, extraction time (required time for achieving the highest extraction performance), salt effect, flow rate of sample solution through the solid phase and breakthrough volume in SPE, were studied in order to maximize the extraction yield of OPPs in the USAL-SPE-LDS-DLLME procedure. Design generation and statistical analysis were performed using the software package STATGRAPHICS Plus version 5.1 for Windows (Rockville, MD, USA).3. Results and discussions
In order to obtain the most effective extraction, an enrichment factor (EF) was used to evaluate the extraction efficiency under different conditions. The enrichment factor was calculated by using eqn (1):
 | (1) |
3.1. Selection of extraction and disperser/eluting solvent
Selection of an appropriate extraction and disperser/eluting solvent is of great importance in an USAL-SPE-LDS-DLLME process. The extraction solvent should be selected on the basis of extraction capability of analytes, gas chromatography behavior and low solubility in water. When combining SPE with LDS-DLLME, the eluting solvent for SPE should also play the role of the disperser solvent at the LDS-DLLME stage. The disperser solvent should be soluble in the extraction solvent and miscible in water, thus enabling the formation of fine droplets of extraction solvent in the aqueous phase. Therefore, toluene, 1-octanol and 1-dodecanol as extraction solvent, and ethanol, acetone and acetonitrile as disperser/eluting solvent were investigated. In Fig. 2, EF of diazinon extraction by using the proposed method is shown for all combinations of disperser/eluting and extraction solvents. This relationship for other OPPs is reported in the ESI section (Fig. S1†). Regarding the EFs, the combination of toluene as the extraction solvent and acetonitrile as the disperser/eluting solvent is the best combination, and EFs between 7215 and 9842 are attainable.3.2. Experimental design
Fractional factorial design (FFD) was employed as a screening design. In this way, the number of experiments was kept low based on the assumption that interaction effects between three or more parameters are small compared to main and two-variable interaction effects. Thus, it is possible to select a fraction of the full fractional design and omit several combinations of parameters from the experimental phase.38,39 The number of experiments in FFD is given by 2k−p + C, where k is the number of variables, C is the number of replicates and p is a whole number that indicates how fractionated the experimental design will be. When p is zero, the experimental design is full.40,41 The investigated factors and their domains are presented in Table 1. Only two levels were used so that the variables were considered as discrete values and no continuous second-order response model could be estimated. High and low levels of each variable are based on the literature34–36 and are designated as −1 and +1.| Variables | Effect symbol | Variable levels | |
|---|---|---|---|
| −1 | +1 | ||
| Volume of extraction solvent (μL) | E | 10 | 20 |
| Volume of disperser/eluting solvent (mL) | D | 0.5 | 1.5 |
| Extraction time (min) | T | 1 | 30 |
| Salt concentration (w/v%) | S | 0 | 5 |
| Flow rate of sample solution through the solid phase (mL min−1) | F | 5 | 30 |
| Volume of sample solution in SPE (mL) | V | 10 | 200 |
The analysis of the results obtained from quarter-fractional factorial design (16 experiments) is visualized using standardized main and two factor interactions effect Pareto charts (P = 95%), as shown in Fig. 3 and Fig. S2.† As shown, the most important factors affecting the USAL-SPE-LDS-DLLE procedure for OPPs determination are volume of disperser/eluting solvent, volume of extraction solvent and flow rate of sample solution through the solid phase. The other variables (salt effect, extraction time and breakthrough volume in SPE) were not significant factors in the studied range.
 | ||
| Fig. 3 Standardized (P = 0.05) Pareto chart representing the estimated effects of parameters and parameter interactions on enrichment factor. | ||
A central composite face centered design for these three factors was applied in order to optimize the level of effective parameters for improving the efficiency of OPPs extraction by USAL-SPE-LDS-DLLME. The total number of design points needed (N) is determined by the following equation:
| N = 2f + 2f + N0 | (2) |
Based on the results of the performed experiments, the second-order polynomial equation was obtained as shown in eqn (3):
| EF = β0 + β1E + β2D + β3F + β11E2 + β12ED + β13EF + β22D2 + β23DF + β33F2 | (3) |
This model consists of three main effects, three two-factor effects and three curvature effects, where β0 is the intercept and β1–β33 terms represent those parameters of the model that are optimized iteratively to fit or model the data. The coefficients of determination (R2 and adjusted-R2) were applied to express the quality of fit of the polynomial model equation. R2 is a measure of the amount of variation around the mean explained by the model. The adjusted-R2 is adjusted for the number of terms in the model. It decreases as the number of terms in the model increases only if those additional terms do not add value to the model. The obtained results for these parameters are listed in Table 2.
| Coefficients | Diazinon | Chlorpyrifos | Thionazin | o,o,o-Triethyl phosphorothioate |
|---|---|---|---|---|
| β0 | 4795.34 | 3717.32 | 4550.9 | 5736.92 |
| β1 | 193.918 | 246.679 | 60.8162 | 80.6266 |
| β2 | 6422.75 | 6370.76 | 4146.62 | 5074.27 |
| β3 | 63.0583 | 70.793 | 33.1132 | 44.2482 |
| β11 | −9.53455 | −11.0516 | −4.5494 | −6.11194 |
| β12 | −31.26 | −28.7998 | −14.4981 | −6.84763 |
| β13 | 1.2216 | 1.12546 | 0.558246 | 0.246741 |
| β22 | −2909.45 | −2907.22 | −1927.34 | −2456.09 |
| β23 | −11.4 | −10.5028 | −4.9682 | −1.69776 |
| β33 | −2.18601 | −2.37675 | −1.22509 | −1.60087 |
| R2 | 95.73 | 93.67 | 97.52 | 98.06 |
| Adjusted-R2 | 91.89 | 87.98 | 95.30 | 96.32 |
In eqn (3), the positive and the negative coefficients of the main effects show how the response changes regarding these variables. The absolute value of a coefficient shows the effectiveness of the related effect. For the graphical interpretation of the interactions, the use of three-dimensional (3D) plots of the model is highly recommended.43–46 Therefore, the results were interpreted based on the 3D graphs obtained from the model. Fig. 4 and S3† show 3D response surfaces and contour plots of the models. The responses were mapped against two experimental factors while the other factor was held constant at its central level.
Fig. 4a and b show that the enrichment factor decreases by increasing the volume of the extraction solvent. This is related to the increase in the volume of the floating organic phase. Increasing the floating organic phase volume leads to decrease of the concentration of OPPs in the floating phase; therefore, the enrichment factor decreases. Fig. 4a and c show that by increasing the disperser/eluting solvent volume in the range of 0.5–1 mL, the efficiency increases. This is because of more efficient elution of the analytes from the SPE cartridge and more proper dispersion of extraction solvent in aqueous solution; however, from 1.0 to 1.5 mL, the efficiency decreases. This behavior can be attributed to the increase of OPPs' solubility in water. The flow rate of the sample solution through the solid phase is an important factor, because it controls the analysis time. On the one hand, the flow rate must be low enough to perform an effective retention of the analytes; on the other hand, it must be high enough not to waste time. As shown in Fig. 4b and c, the maximum enrichment factor was observed in the flow rate of 14–15 mL min−1. It was low enough to perform an effective extraction and high enough to shorten the time reasonably.
After the analysis of the results, the following conditions were selected as optimal working conditions to evaluate the performance of the extraction procedure for organophosphorus pesticides: 10 μL of toluene as extraction solvent, 1 mL of acetonitrile as dispersive/elution solvent, 1 min as extraction time, 0 w/v% of salt, 15 mL min−1 as flow rate of sample solution through the solid phase and 50 mL of sample for SPE.
3.3. Analytical performance
The proposed method was evaluated under the optimum condition for linearity, precision, limits of detection (LODs), limits of quantification (LOQs) and EFs. The results are summarized in Table 3. The linear dynamic ranges (LDRs) were obtained in the range of 0.012–625 ng g−1 and the squared regression coefficients (R2) exceeded 0.9931 for all analytes. LODs of the entire method (based on S/N = 3) for the selected analytes ranged from 0.002 to 0.125 ng g−1 and LOQs (S/N = 10) from 0.007 to 0.4 ng g−1, which seemed quite promising for trace analysis of the analytes in soil samples. The relative standard deviations (RSDs%) for extraction and determination of the analytes were less than 8.3% based on six replicates. The enrichment factors ranged between 7215 and 9842.| Analyte | Linearity | LOD (ng g−1) | LOQ (ng g−1) | Precision (RSD%, n = 6) | EFa | |
|---|---|---|---|---|---|---|
| LDR (ng g−1) | R2 | |||||
| a Extraction factors were calculated based on extraction of 50 ng g−1 of each OPP. | ||||||
| Diazinon | 0.012–625 | 0.9987 | 0.002 | 0.007 | 7.10 | 9842 |
| Chlorpyrifos | 0.025–625 | 0.9931 | 0.012 | 0.04 | 6.80 | 8799 |
| Thionazin | 0.25–625 | 0.9955 | 0.125 | 0.4 | 8.30 | 7215 |
| o,o,o-Triethyl phosphorothioate | 0.025–625 | 0.9963 | 0.005 | 0.017 | 5.40 | 9024 |
3.4. Comparison of the proposed method with other methods
The efficiency of the represented USAL-SPE-LDS-DLLME method was compared with the previously reported methods for determination of OPPs in soil samples. The details of comparison are summarized in Table 4. With respect to other methods, the proposed method has very low LODs and extremely high EFs. The time required to complete the extraction process, the sample consumption and the linearity obtained by the USAL-SPE-LDS-DLLME are comparable to or better than other reported methods. Moreover, in our proposed method, low density, low toxicity and more environmentally friendly organic solvents were used as extraction solvents. Considering the results, this method proved to be a rapid, sensitive, repeatable and easy to use technique in the determination of OPPs in soil samples.| Method | LODa (ng g−1) | RSDb (%) | Enrichment factor | Timec (min) | Sample consumption (g) | LDR (ng g−1) | Reference |
|---|---|---|---|---|---|---|---|
| a Limit of detection.b Relative standard deviation.c The required time for complete extraction process.d Supercritical fluid extraction.e Microwave-assisted extraction.f Single drop microextraction.g Ultrasound assisted emulsification microextraction.h Modified matrix solid-phase dispersion.i Ultrasound leaching-solid phase extraction-dispersive-solidification liquid–liquid microextraction. | |||||||
| SFEd-DLLME-GC-FID | 1–9 | 3.1–7.5 | 97–144 | 40 | 1.2 | 20–8300 | 2 |
| MAEe-GC-MS | 0.10–0.12 | 2.5–9.4 | — | 10 | 1 | 1–250 | 4 |
| SDMEf-GC-NPD | 0.1–2 | 2.1–6.9 | 1.4–12.7 | 11 | 2 | 10–300 | 7 |
| MAE/USAEMEg-GC-ECD | 0.04–0.13 | 3.1–8.2 | — | 8–9 | 1 | 0.25–10 | 15 |
| MMSPDh-GC-NPD | 0.1–0.6 | 1.6–4.5 | — | 60 | 10 | — | 47 |
| USL-SPE-DSLLMEi-GC-MS | 0.012–0.2 | 4.06–8.9 | 6890–8830 | 15 | 2 | 0.025–625 | 48 |
| USAL-SPE-LDS-DLLME-GC-MS | 0.002–0.125 | 5.4–8.3 | 7215–9842 | 15 | 2 | 0.012–625 | This work |
3.5. Soil sample analysis
The practical applicability of the proposed USAL-SPE-LDS-DLLME method was evaluated under the optimized conditions by extracting the selected organophosphorus pesticides from several soil samples. The concentrations of diazinon, chlorpyrifos, thionazin and o,o,o-triethyl phosphorothioate in all of the soil samples were detected. To assess matrix effects, the soil samples were spiked with the four OPPs standards at the concentration of 50 ng g−1. Three replicate experiments using the whole analysis process were performed and the results are given in Table 5. The relative recovery (RR) was obtained by the following equation:
 | (4) |
| Sample | 1a | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| a (1): Diazinon; (2): chlorpyrifos; (3): thionazin; (4): o,o,o-triethyl phosphorothioate.b I: cucumber farm soil; II: cabbage farm soil; III: flat soil; IV: citrus soil; V: greenhouse soil.c All concentrations are in ng g−1.d Concentration of OPPs in spiked samples, which was found by the proposed method.e 50 ng g−1 of each OPP was spiked in soils. | |||||
| Soil Ib | Initial concentrationc | 223.66 | 208.17 | 28.93 | 28.49 |
| Foundd,e | 272.87 | 254.98 | 73.91 | 75.58 | |
| Relative recovery (%) | 98.42 | 93.63 | 89.96 | 94.18 | |
| RSD% | 7.9 | 8.2 | 9.7 | 7.6 | |
| Soil II | Initial concentration | 175.25 | 172.70 | 14.44 | 7.33 |
| Found | 219.42 | 219.49 | 64.17 | 53.25 | |
| Relative recovery (%) | 88.34 | 93.58 | 99.47 | 91.85 | |
| RSD% | 6.7 | 5.9 | 6.3 | 5.1 | |
| Soil III | Initial concentration | 4.53 | 12.53 | 3.59 | 1.17 |
| Found | 53.74 | 60.41 | 50.45 | 48.57 | |
| Relative recovery (%) | 98.43 | 95.76 | 93.72 | 94.81 | |
| RSD% | 8.2 | 7.8 | 6.3 | 7.5 | |
| Soil IV | Initial concentration | 10.58 | 18.62 | 5.22 | 3.81 |
| Found | 55.94 | 62.92 | 52.01 | 49.88 | |
| Relative recovery (%) | 90.73 | 88.61 | 93.59 | 92.15 | |
| RSD% | 8.1 | 7.5 | 5.9 | 6.1 | |
| Soil V | Initial concentration | 63.67 | 91.01 | 8.72 | 2.49 |
| Found | 112.47 | 138.80 | 52.61 | 50.02 | |
| Relative recovery (%) | 97.61 | 95.58 | 87.79 | 95.07 | |
| RSD% | 5.8 | 6.3 | 4.2 | 5.7 | |
The relative recoveries of the four analytes were satisfactory in the range between 87.79% and 99.47%. These values of recoveries have confirmed the validity of the proposed method.
4. Conclusion
The proposed analytical methodology based on the USAL-SPE-LDS-DLLME technique is an efficient alternative for OPPs determination at trace levels in soil samples. The combination of USAL-SPE led to an increment of methodology, selectivity and sensitivity; it explains the LDS-DLLME preconcentration capabilities for complex soil samples. Toluene (LDS), which is relatively less toxic in comparison with the widely used chlorinated solvents in DLLME, was successfully used in the present approach. It is important to highlight that the experimental design helped to identify and characterize the variables and their interactions that govern the system. This statistical tool also allowed reaching optimum working condition with a minimum number of analytical assays. The main benefits of the proposed methodology for extraction and determination of OPPs were low sample consumption, minimum use of toxic organic solvent, satisfactory extraction time, rejection of matrix constituent, simplicity and high enrichment factor. Therefore, the proposed approach can be successfully applied in routine analysis to determine low concentration levels of OPPs in soil samples.References
- Z. Yang, Y. Liu, D. Liu and Z. Zhou, J. Chromatogr. Sci., 2012, 50, 15–20 CAS.
- M. H. Naeeni, Y. Yaminia and M. Rezaee, J. Supercrit. Fluids, 2011, 57, 219–226 CrossRef CAS PubMed.
- J. A. A. Castilho, N. Fenzl, S. M. Guillen and F. S. Nascimento, Environ. Pollut., 2000, 110, 523–533 CrossRef CAS.
- Y. Merdassa, J. Liu and N. Megersa, Talanta, 2013, 114, 227–234 CrossRef CAS PubMed.
- X. Mao, Y. Wan, A. Yan, M. Shen and Y. Wei, Talanta, 2012, 97, 131–141 CrossRef CAS.
- M. V. Russo, P. Avino, G. Cinelli and I. Notardonato, Anal. Bioanal. Chem., 2012, 404, 1517–1527 CrossRef CAS PubMed.
- A. Salemi, R. Rasoolzadeh, M. Mohebbi Nejad and M. Vosough, Anal. Chim. Acta, 2013, 769, 121–126 CrossRef CAS PubMed.
- M. J. Melgar, M. Santaeufemia and M. A. Garcia, J. Environ. Sci. Health, Part B, 2010, 45, 595–600 CrossRef CAS.
- S. Samadi, H. Sereshti and Y. Assadi, J. Chromatogr. A, 2012, 1219, 61–65 CrossRef CAS PubMed.
- Q. Liu, W. Kong, F. Qiu, J. Wei, S. Yang, Y. Zheng and M. Yang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2012, 885–886, 90–96 CrossRef CAS PubMed.
- S. N. Sinha, M. V. Vardhana Rao, K. Vasudev and M. Odetokun, Food Control, 2012, 25, 636–646 CrossRef CAS PubMed.
- M. Ebrahimi, Z. Es'haghi, F. Samadi, F. F. Bamoharram and M. S. Hosseini, J. Chromatogr. A, 2012, 1225, 37–44 CrossRef CAS PubMed.
- T. M. Gutiérrez Valencia and M. P. García de Llasera, J. Chromatogr. A, 2011, 1218, 6869–6877 CrossRef PubMed.
- C. Gonçalves, J. J. Carvalho, M. A. Azenha and M. F. Alpendurada, J. Chromatogr. A, 2006, 1110, 6–14 CrossRef PubMed.
- Y. Su, C. Yan, V. K. Ponnusamy and J. Jen, J. Sep. Sci., 2013, 36, 2339–2347 CrossRef CAS PubMed.
- D. Sanyal, A. Rani, S. Alam, S. Gujral and R. Gupta, Environ. Monit. Assess., 2011, 182, 97–113 CrossRef CAS PubMed.
- A. Hildebrandt, S. Lacorte and D. Barceló, Anal. Bioanal. Chem., 2007, 387, 1459–1468 CrossRef CAS PubMed.
- R. Shi, J. Lv and J. Feng, Bull. Environ. Contam. Toxicol., 2011, 87, 567–573 CrossRef CAS PubMed.
- M. Asensio-Ramos, J. Hernández-Borges, T. M. Borges-Miquel and M. A. Rodríguez-Delgado, Anal. Chim. Acta, 2009, 647, 167–176 CrossRef CAS PubMed.
- R. D. Durovic, T. M. Dordevic, L. R. Santric, S. M. Gasic and L. M. Ignjatovic, J. Environ. Sci. Health, Part B, 2010, 45, 626–632 CrossRef CAS PubMed.
- Y. Abdollahzadeh, Y. Yamini, A. Jabbari, A. Esrafili and M. Rezaee, Anal. Methods., 2012, 4, 830–837 RSC.
- N. B. Lana, P. Berton, A. Covaci, A. G. Atencio, N. F. Ciocco and J. C. Altamirano, J. Chromatogr. A, 2013, 1285, 15–21 CrossRef CAS PubMed.
- A. R. Fontana, N. B. Lana, L. D. Martinez and J. C. Altamirano, Talanta, 2010, 82, 359–366 CrossRef CAS PubMed.
- M. Rezaee, Y. Assadi, M. R. M. Hosseini, E. Aghaee, F. Ahmadi and S. Berijani, J. Chromatogr. A, 2006, 1116, 1–9 CrossRef CAS PubMed.
- F. Vela-Soria, O. Ballesteros, A. Zafra-Gómez, L. Ballesteros and A. Navalón, Talanta, 2014, 129, 209–218 CrossRef CAS.
- M. L. Martins, O. D. Prestes, M. B. Adaime and R. Zanella, Anal. Methods, 2014, 6, 5020–5027 RSC.
- N. M. Najafi, H. Tavakoli, R. Alizadeh and S. Seidi, Anal. Chim. Acta, 2010, 670, 18–23 CrossRef CAS PubMed.
- M. A. Farajzadeh, S. E. Seyedi, M. S. Shalamzari and M. Bamorowat, J. Sep. Sci., 2009, 32, 3191–3200 CrossRef CAS PubMed.
- N. M. Najafi, H. Tavakoli, Y. Abdollahzadeh and R. Alizadeh, Anal. Chim. Acta, 2012, 714, 82–88 CrossRef CAS PubMed.
- M. Asadollahzadeh, N. Niksirat, H. Tavakoli, A. Hemmati, P. Rahdari, M. Mohammadi and R. Fazaeli, Anal. Methods, 2014, 6, 2973–2981 RSC.
- A. Saleh, Y. Yamini, M. Faraji, M. Rezaee and M. Ghambarian, J. Chromatogr. A, 2009, 1216, 6673–6679 CrossRef CAS.
- M. Asadollahzadeh, H. Tavakoli, M. Torab-Mostaedi, G. Hosseini and A. Hemmati, Talanta, 2014, 123, 25–31 CrossRef CAS PubMed.
- H. Cabuk, M. Akyuz and S. Ata, J. Sep. Sci., 2012, 1–8 Search PubMed.
- S. Zhou, H. Chen, B. Wu, C. Ma and Y. Ye, Microchim. Acta, 2012, 176, 419–427 CrossRef CAS.
- L. Guo and H. K. Lee, J. Chromatogr. A, 2013, 1300, 24–30 CrossRef CAS PubMed.
- B. M. Liu, H. Y. Yan, F. X. Qiao and Y. R. Geng, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 90–94 CrossRef CAS PubMed.
- L. Sun and H. K. Lee, J. Chromatogr. A, 2003, 1014, 165–177 CrossRef CAS.
- R. G. Brereton, Chemometrics – Data Analysis for the Laboratory and Chemical Plant, Wiley, Chichester, 2003, pp. 76–77 Search PubMed.
- M. Preu, D. Guyot and M. Petz, J. Chromatogr. A, 1998, 818, 95–108 CrossRef CAS.
- E. Martendal, D. Budziak and E. Carasek, J. Chromatogr. A, 2007, 1148, 131–136 CrossRef CAS PubMed.
- L. V. Candioti, J. C. Robles, V. E. Mantovani and H. C. Goicoechea, Talanta, 2006, 69, 140–147 CrossRef CAS PubMed.
- S. C. L. Ferreira, W. N. L. dos Santos, C. M. Quintella, B. B. Neto and J. M. Bosque-Sendra, Talanta, 2004, 63, 1061–1067 CrossRef CAS.
- R. L. Mason, R. F. Gunst and J. J. Hess, Statistical Design and Analysis of Experiments with Applications to Engineering and Science, Wiley, New York, 2003 Search PubMed.
- D. C. Montgomery, G. C. Runger and N. F. Hubele, Engineering Statistics, Wiley, New York, 2001 Search PubMed.
- G. G. Vining, Statistical Methods for Engineers, Duxburg Press, London, 2003 Search PubMed.
- H. Sereshti, M. Karimi and S. Samadi, J. Chromatogr. A, 2009, 1216, 198–204 CrossRef CAS.
- X. Shen, J. Cai, Y. Gao and Q. Su, Chromatographia, 2006, 64, 71–77 CAS.
- K. Ahmadi, Y. Abdollahzadeh, M. Asadollahzadeh, A. Hemmati, H. Tavakoli and R. Torkamand, Talanta, 2015, 137, 167–173 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11959f |
| This journal is © The Royal Society of Chemistry 2015 |