Modeling of biosynthesized silver nanoparticles in Vitex negundo L. extract by artificial neural network
Abstract
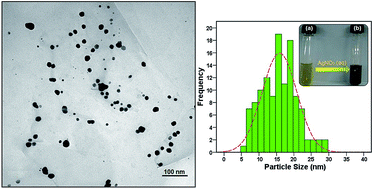
In this study silver nanoparticles (Ag-NPs) are biosynthesized from silver nitrate aqueous solution through a simple and eco-friendly route using water extract of Vitex negundo L. (V. negundo) which acted as a reductant and stabilizer simultaneously. The as prepared samples are characterized using UV-visible spectroscopy, X-ray diffraction (XRD), and transmission electron microscopy (TEM). Also artificial neural network (ANN) model was presented for synthesized silver nanoparticles in V. negundo L. extract. The aim was to predict size of silver nanoparticles produced as a function of the weight percentage of V. negundo L. extract, reaction of temperature, stirring time and molar concentration of AgNO3. The fast Levenberg–Marquardt (LM) optimization technique was employed for training of ANN model. The optimized ANN was as a multilayer perceptron (MLP) network with two hidden layers and 10 neurons. Therefore ANN is found out to be an efficient tool to model the complicated chemical field. This model is capable for predicting the size of nanoparticles for a wide range of conditions with a mean square error 0.4576 and a regression of about 0.998. Based on the presented model it is possible to design an effective green method for obtain silver nanoparticles, while minimum received materials are used and minimum size of nanoparticles will be obtained.

 Please wait while we load your content...
Please wait while we load your content...