A simple and rapid approach for testing enantiopurity of hydroxy acids and their derivatives using 1H NMR spectroscopy†
Abstract
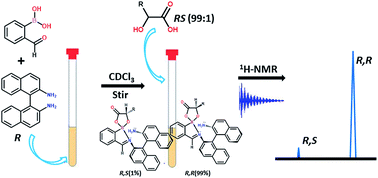
A rapid and the simple chiral derivatizing protocol involving the coupling of 2-formylphenylboronic acid and an optically pure [1,1-binaphthalene]-2,2-diamine is introduced for the accurate determination of the enantiopurity of hydroxy acids and their derivatives, possessing one or two optically active centers, using 1H NMR spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...