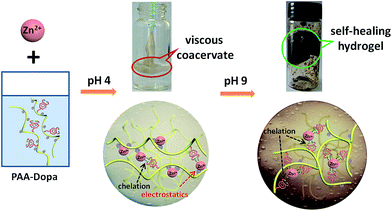
Zinc induced polyelectrolyte coacervate bioadhesive and its transition to a self-healing hydrogel†
Weina Wang‡
ab,
Yisheng Xu‡*a,
Ang Lia,
Tao Lia,
Miaomiao Liua,
Regine von Klitzingb,
Christopher K. Oberc,
A. Basak Kayitmazerd,
Li Lia and
Xuhong Guo*ae
aState Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: yshxu@ecust.edu.cn; guoxuhong@ecust.edu.cn
bStranski-Laboratorium für Physikalische und Theoretische Chemie, Technische Universität Berlin, Strasse des 17. Juni 124, D-10623 Berlin, Germany
cDepartment of Materials Science and Engineering, Cornell University, Ithaca, NY 14853-1501, USA
dChemistry Department, Bogazici University, Bebek, Istanbul 34342, Turkey
eKey Laboratory of Materials-Oriented Chemical Engineering of Xinjiang Uygur Autonomous Region and Engineering Research Center of Materials-Oriented Chemical Engineering of Xinjiang Bingtuan, Shihezi University, Xinjiang 832000, China
First published on 30th July 2015
Abstract
To mimic the underwater adhesion of marine mussels, a bioadhesive has been prepared with a poly(acrylic acid) backbone functionalized with 30% catechol appendants. The polyelectrolyte chains can be reversibly crosslinked through metal chelation and irreversibly gelled by oxidative crosslinking. Surprisingly, the reported “poor” metal chelator Zn2+ not only imparts this injectable adhesive with superior adhesion after the formation of coacervation compared to the one chelated by a stronger metal crosslinker (e.g. Fe3+), but also generates good mechanical performance of the self-healing hydrogel after the oxidation of catechol groups with a pH trigger. Such a pH-responsive material with strong adhesion and good self-healing property at different conditions could be an ideal candidate in biomedical adhesion and tissue engineering.
1. Introduction
The development of novel biomaterials plays an essential role in the successful use of wound sealants in surgery. However an ideal wound sealant has been a challenging topic for decades because the tissue engineering process requires a biocompatible material with excellent wettability to be injected and spread on the wound precisely. In addition effective curing and adequate mechanical properties are indispensable to maintain stability in situ. Dopa-modified polymeric structures inspired by marine organisms such as mussels and sandcastle worms living in a harsh seawater environment have garnered increasing interest to explore biomimetic adhesives for tissue engineering or other medical purposes particularly for use in wet environments. The key functional groups are catechol side chains which can be easily crosslinked by metal ions1 to form catechol complexes (mono-, bis-, and tris-complex). On the other hand, such structures can also undergo chemical/enzymatic oxidation2–4 to form stable covalent bonds with bidentate H-groups5 and thus possess enough cohesive force for good performance adhesives.A number of recent investigations have focused on the improvement of adhesive properties of bioadhesives using metal chelated hydrogels probably because of the difficulty of using strong oxidants in biological systems. The metal chelated catechol system were proposed to be ideal candidates for tissue adhesive and sealant.6,7 Some alternative backbones have been used such as poly(ethylene glycol) (PEG),7,8 polystyrene9 or hyaluronic acid (HA)10 to enhance adhesive performance. These polymers demonstrate performance competitive to commercial super glues after crosslinking even though they exhibit very dark color of the materials after curing. Nevertheless, the fact that metal ions harden the material gives self-healing ability on the bulk scale which somehow limits the adhesive performance of the materials (several hundred kPa level).6 As noticed,6,11 the oxidation process reduces adhesive force and provides enough cohesive force for adhesives, while metal chelation strongly enlarges the cohesive force range, hence facilitating the formation of hydrogels.
In this work, a novel water-based polymeric adhesive with good bonding strength and fast in situ sealing capabilities is presented as effective tissue adhesive and orthopedic sealant.12 Interestingly, this material is based on a delicate Dopa functionalized poly(acrylic acid) in the presence of weak divalent cross-linker Zn2+. Zn2+ was rarely used in Dopa containing polymers since it showed little crosslinking in most polymeric backbone system.13 In current study, poly(carboxylic acid) such as poly(acrylic acid) (PAA) was chosen as the backbone because of its high sorption capacity to chelate metal ions14 and the highly soluble electrolytic property which can take place of the protein polyamide chain of mussel adhesive protein. Since mussels concentrate Zn2+ more than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold higher in the adhesive plaque compared to its concentration in sea water.15 In addition, zinc supplementation has been found to improve wound healing in zinc-deficient cases,16 Zn2+ was hence chosen to be the metal chelator. In the absence of catechol groups, complex formation of Zn2+ with PAA by electrostatics in an aqueous acidic environment (low pH) shows no adhesion but a turbid solution.17 By partially conjugating catechol groups to PAA backbone, the anionic characteristics of the polymers are retained. Taking advantage of “sticky” features of the catechol group, the adhesive force can be maximized when a coacervate is created from PAA-Dopa in the presence of Zn2+ at low pH (Scheme 1).
000-fold higher in the adhesive plaque compared to its concentration in sea water.15 In addition, zinc supplementation has been found to improve wound healing in zinc-deficient cases,16 Zn2+ was hence chosen to be the metal chelator. In the absence of catechol groups, complex formation of Zn2+ with PAA by electrostatics in an aqueous acidic environment (low pH) shows no adhesion but a turbid solution.17 By partially conjugating catechol groups to PAA backbone, the anionic characteristics of the polymers are retained. Taking advantage of “sticky” features of the catechol group, the adhesive force can be maximized when a coacervate is created from PAA-Dopa in the presence of Zn2+ at low pH (Scheme 1).
The interactions between PAA-Dopa and Zn2+ can be manipulated by external conditions to sequentially form high performance coacervate adhesive (strong adhesion, electrostatic interactions) to hydrogel (good stress recovery, coordinate and covalent interactions) in a simple process. The optimization of the cohesive and adhesive forces imparts the material with both hardness and adhesive properties.11 In principle, such complex coacervate adhesive can instantly form after being injected at low pH and then gelled at elevated pH. This system is probably ideal for tissue repair even though many practical issues still need to be carefully considered from the aspect of medical purposes.
2. Experimental
2.1 Materials
Poly(acrylic acid) (PAA) (Mw = 250 kg mol−1, 35 wt% in H2O), dopamine hydrochloride, 1-methyl-2-pyrrolidinone (NMP) (99.5%), N,N′-dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS) (98%), sodium tetraborate (Na2B4O7), sodium bicarbonate (NaHCO3), ethyl acetate (C4H8O2), zinc chloride (ZnCl2) were purchased from Sigma Aldrich. PAA solution was freeze dried and ground to white powder before experiments. Other chemicals were used without further purification.2.2 Synthesis and purification of PAA-Dopa
The synthesis was based on our previous report.18 Because of the highly reactive property of dopamine moiety, dopamine hydrochloride was initially stored under moderately basic conditions in an aqueous solution of sodium borate and sodium bicarbonate, where the medium protected dopamine moiety from side reactions by forming borate ester.19 After grafting, the pH of aqueous solution was reduced to less than pH 2 in order to deprotect the dopamine moiety. PAA-Dopa was then extracted with ethyl acetate.20 This synthesis was carried out under nitrogen. The product of PAA-Dopa powder is water-soluble. In a typical run, PAA (1.0 g, 13.9 mmol –COOH groups) was dissolved in NMP (20 ml) at 60 °C for 24 h. DCC (0.587 g, 2.845 mmol) and NHS (0.058 g, 0.5 mmol) were introduced into the PAA solution under magnetic stirring for 2 h for the following use. Na2B4O7 (1.144 g, 5.69 mmol) and NaHCO3 (0.478 g, 5.69 mmol) were dissolved NMP (30 ml) under vigorous stirring and bubbled with N2 for 0.5 h. Dopamine hydrochloride (1.841 g, 9.71 mmol) was then added for another 0.5 h mixing, followed by dropwise addition of well-prepared PAA mixture. The reaction mixture was stirred overnight at room temperature with N2 bubbling. Finally, the pH of reaction solution was reduced to less than 2 and precipitated three times with ethyl acetate (250 ml in total). For further purification, the crude wet product was dissolved into DI water (30 ml) and dialyzed (SpectraPor 3 tubing, molecular weight cutoff: 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) against DI water in dark until the conductivity of water outside the bag reached constant. The final product was freeze dried to get PAA-Dopa powder which was then stored in a sealed container.
000 g mol−1) against DI water in dark until the conductivity of water outside the bag reached constant. The final product was freeze dried to get PAA-Dopa powder which was then stored in a sealed container.
2.3 Preparation of Zn2+ crosslinked coacervate/hydrogel
PAA-Dopa with a catechol percentage of ∼30 mol% was dissolved in pH 4 acetate buffer (0.35 M of Dopa). Zn2+ solution with different concentration was introduced into PAA-Dopa solution with the same volume. Coacervate was instantly formed. At pH ∼ 9 this material was triggered the formation of self-healing hydrogel. For better comparison of hydrogel with different crosslinking degrees, these pH triggered hydrogels were then allowed to settle for 1–3 hours in an air tight container to facilitate complete diffusion and relaxation of the polymer network.2.4 UV-vis spectroscopy with PAA–catechol and Zn2+
Dopamine grafting stoichiometry was determined in pH 4 acetate buffer with an SHIMADZU 2550 spectrophotometer using a 1 cm path length quartz cuvette. The catechol group displays a characteristic peak at λ279 nm (λ279 = 2600 M−1 cm−1).21 Same volume of PAA-Dopa (0.35 M, pH 4 buffer) and Zn2+ (with stoichiometric Zn2+/Dopa of 1.4, 3.2, 4.0, and 8.0, respectively) were mixed and magnetically stirred for 2 h and centrifuged, after which certain amount of the supernatant was diluted with deionized water to keep λmax inside the linear absorption range (λmax < 1).2.5 Rheology
All rheological measurements were performed by MCR501 rheometer from Anton-Paar Physical (Austria) equipped with the 25 mm parallel plate geometry. In brief, same volume of Zn2+ and PAA-Dopa (0.35 M, pH 4) solutions were added onto the rheometer baseplate. Water-born adhesives formed after gentle stirring for a few seconds. Soft adhesives were extruded on the platform. To study the transition temperature of water-born adhesives with different cross-linking degree (Zn2+ concentration of 0.5, 0.7, and 1.4 M), measurements were performed at a frequency of 1 Hz and a strain of 1% and the temperature was ramped from 25 to 45 °C at a rate of 0.5 °C min−1. To investigate the stable self-healing property, strain was increased from 0.1 to 1000% in 200 s to break the structure of hydrogel after which the recovery process was monitored by small amplitude oscillation at 1% strain for another 200 s. The strain-recovery tests were repeated five times. To compare the dynamic rheological properties between coacervates and hydrogels, and by extension, the nature of the metal–ligand crosslinks, frequency sweeps were performed under different crosslinking degree in the linear viscoelastic range at constant strain amplitude of 1% from 1 to 100 rad s−1 at room temperature.2.6 Lap shear adhesion testing
Lap-shear adhesion tests were conducted with a Hengyi HY-0580 tensile test instrument with a 3000 N loading cell. The loading rate was 5 mm min−1 to reach an ultimate force for all samples. Different adherends (1 cm × 2 cm) of aluminum, stainless steel, polyethylene (PE) and Teflon were used for these tests. Adherends were first polished with aluminum oxide cloth (120#) followed by washing with hexanes, ethanol, acetone, deionized water and finally air-dried overnight. Additional washes and scratches have to be employed to get rid of insoluble residues if necessary. Zinc chloride solution (200 μl, 1.4 M) was evenly spread over one adherend, followed by PAA-Dopa solution (200 μl, 0.35 M of catechol group) on top of it. Two adherends were overlapped to fulfill an area of 1 cm × 2 cm, pressed together squeezing out a small excess of adhesive and clamped. For each sample, 5 trials were conducted. For dry curing method, samples were first pressed for 1 h at room temperature (20 °C). Then they were cured at 40 °C for 22 h and finally cooled at room temperature (20 °C) for 1 h.22 For semi-wet curing method, samples prepared above were subsequently stored underwater at room temperature for another day. For wet curing method, fresh bonded specimens with water-born adhesives were immediately incubated underwater at room temperature for 24 h. The shear strength was immediately measured after the samples were taken out of water. A widely used commercial α-cyanoacrylic resin adhesive was used by simple mixing A, B glues together (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight, 15 mg in total) for comparison.
1 by weight, 15 mg in total) for comparison.
2.7 Other characterizations
The morphology of coacervate droplets was examined with a LEIKA DM 2500P PLM. Images were captured with a charge-coupled device camera connected to a PC via a WT-1000GM imaging board. FT-IR spectra were obtained with a Nicolet 6700 instrument (Nicolet Instrument, Thermo Company, USA) over the wave number range of 4000–1000 cm−1. 1H-NMR spectra were recorded on a BRUKER AVANCE 500 spectrometer operating at 500 MHz with D2O as solvent. SEM results of surface morphology of lyophilized adhesives and hydrogels were obtained by NOVA Nano SEM 450 with an accelerating voltage of 3 keV. Before analysis, a thin Au film was coated for conductivity.3. Results and discussion
3.1 Synthesis of catechol modified polymer
Synthesis of PAA-Dopa was illustrated in Scheme 2. The PAA used here has an average molecular weight of 250 kDa. The appearance of a stretching peak at 1645 cm−1 and bending peak at 1544 cm−1 by transmission infrared spectroscopy (Fig. S1, ESI†) indicates the successful attachment of dopamine groups. Approximately 30% of carboxylate groups were grafted with dopamine through an amidation reaction demonstrated by NMR (Fig. S2, ESI†). Furthermore, the adjustment of the adhesive and cohesive force by pH can induce a transition of adhesive to hydrogel in a simple Dopa–metal system in the presence of Zn2+. The resulted adhesive demonstrates strong adhesion in both dry and aqueous states with no need of strong oxidants compared to the same type of underwater adhesives currently reported.10,21–23From UV-vis spectra, the characteristic peak of the hydroxyl moiety at 279 nm (ref. 21) was used to estimate the grafting degree of catechol groups on the PAA backbone as shown in Fig. S3a, ESI.† As noticed, Dopa groups grafted on the PAA chains remain in their reduced states without being oxidized: no shoulder between 310 and 380 nm was observed implying no formation of α,β-dehydro Dopa intermediates.24 This means good protection of the reactions from oxygen. The grafting density ranges from 15% to 40% as the Dopa/AA ratio increases from 0.2 to 1 (Fig. S3a and b, ESI†). In this work, 30% of grafting density was chosen because this ratio is consistent to the catechol content in foot protein 5 from the common blue mussel (M. edulis)25 which contains the highest Dopa content among all marine adhesive proteins.
3.2 Zinc induced electrostatically driven coacervation
At fixed Dopa grafting ratio (30%), PAA-Dopa solution was injected into zinc solution at different concentrations. Dense coacervate was immediately formed (Video, ESI†). As the zinc concentration was increased, the free catechol groups on PAA chains in the supernatant decreased reflecting the increase of catechol chelation degree (Fig. S3c and d, ESI†). However, the free catechol concentration did not change as the Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dopa ratio is greater than 4. The resulted adhesives exclusively manifest high viscosity and grey color. The results here are somehow different from previous results found in Fe3+ systems in which mono-catechol–Fe3+ complexes dominate and no crosslinking exists at low pH conditions.26,27 Since Zn2+ has a lower charge density than iron as a consequence of its larger size and lower charge, weaker complexation with catechol groups is expected to form mono-catechol–Zn2+ complexes. Meanwhile, zeta potentials of PAA and PAA-Dopa are −16.8 and −22.6 mV respectively (Fig. S4†) at pH 4 where quite a few carboxylic groups are dissociated and negatively charged, hence it is more likely to have electrostatic interactions during the formation of coacervate. It is believed that the electrostatic interaction between positively charged Zn2+ (chelated with catechol groups) and negatively charged carboxylic groups from another polymer chain can certainly facilitate the formation of inter-polymeric complexes (Scheme 1) and hence certain cohesive force was reached.
Dopa ratio is greater than 4. The resulted adhesives exclusively manifest high viscosity and grey color. The results here are somehow different from previous results found in Fe3+ systems in which mono-catechol–Fe3+ complexes dominate and no crosslinking exists at low pH conditions.26,27 Since Zn2+ has a lower charge density than iron as a consequence of its larger size and lower charge, weaker complexation with catechol groups is expected to form mono-catechol–Zn2+ complexes. Meanwhile, zeta potentials of PAA and PAA-Dopa are −16.8 and −22.6 mV respectively (Fig. S4†) at pH 4 where quite a few carboxylic groups are dissociated and negatively charged, hence it is more likely to have electrostatic interactions during the formation of coacervate. It is believed that the electrostatic interaction between positively charged Zn2+ (chelated with catechol groups) and negatively charged carboxylic groups from another polymer chain can certainly facilitate the formation of inter-polymeric complexes (Scheme 1) and hence certain cohesive force was reached.
The formation of complex adhesive and the condensation from aqueous solution can be identified from Fig. 1 through visual and microscopic observations. The rapid progress of such complexation leads to further neutralization of the complex and finally to the formation of coacervate in the presence of Zn2+. This also explains the previous findings that Zn2+ often promotes hardness properties when mixed with polycarboxylate polymers.13 As a comparison, addition of Zn2+ to simple PAA or Dopa solution does not form viscous liquid phase or provides any adhesion. Without the negatively charged structure and the sticky sidechain, the material does not generate enough cohesive or adhesive force to function as an adhesive.
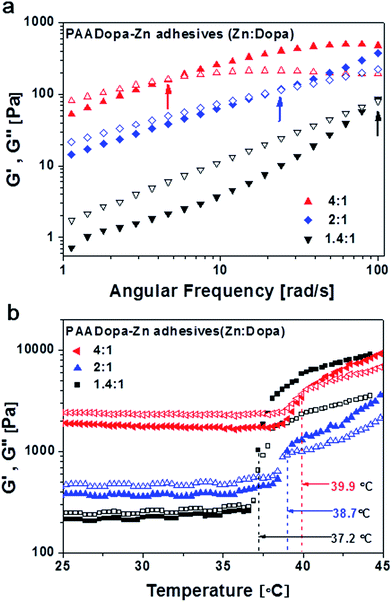
A lower elastic modulus (G′) than viscous modulus (G′′) determined by rheology analysis (Fig. 2a) indicates fluidic properties at low pH (∼4) especially at low Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dopa ratios. The curves of G′ and G′′ as a function of frequency crossed each other, and the cross points shifts to lower frequency as the Zn
Dopa ratios. The curves of G′ and G′′ as a function of frequency crossed each other, and the cross points shifts to lower frequency as the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dopa ratio was raised. This means that the relaxation time τ* (τ* = 1/f*) becomes longer, where f* is the crossover frequency at G′ = G′′. The τ* suggests the time scale of the mechanical relaxation in the polymeric structure. A larger τ* reflects a stronger attractive interactions between polymer chains28 as expected since more cationic Zn added facilitates both chelation and coulombic interactions.
Dopa ratio was raised. This means that the relaxation time τ* (τ* = 1/f*) becomes longer, where f* is the crossover frequency at G′ = G′′. The τ* suggests the time scale of the mechanical relaxation in the polymeric structure. A larger τ* reflects a stronger attractive interactions between polymer chains28 as expected since more cationic Zn added facilitates both chelation and coulombic interactions.
In Fig. 2b, the temperature dependence of G′ and G′′ was investigated to reveal the phase transition behaviors of such adhesive. Three kinds of samples with different zinc ratios were studied and the cross points of G′ and G′′ can be clearly observed as the samples transform from liquid-like to gel-like samples. The overall response to temperature is between 37 and 40 °C likely suggesting a superior intrinsic feature for physiological temperature response. An enhancement of sample stoichiometry (Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dopa) does not increase the transition temperature dramatically. Under conditions of the test set-up, the possible oxidative crosslinking is excluded by the remained light color of the adhesives. Therefore the transition temperatures are mainly due to the physical phase change of the adhesive.21
Dopa) does not increase the transition temperature dramatically. Under conditions of the test set-up, the possible oxidative crosslinking is excluded by the remained light color of the adhesives. Therefore the transition temperatures are mainly due to the physical phase change of the adhesive.21
The quantitative lap shear mechanical analysis (as shown in Fig. S5†) of adhesive strength in both dry and wet states was performed on different types of substrates including aluminum, stainless steel, polyethylene (PE) and Teflon. The results are presented in Fig. 3a and b. In general, adhesives on rough metal surfaces with high free energy perform much better and this is similar to the cases of mussel adhesion on different substrates.22,29 In the curing process, a temperature (40 °C) near physiological temperature was chosen as the curing temperature and samples were cured for at least 24 hours to ensure the complete solidification on all substrates. After curing, Dopa groups in all samples were automatically aerobic oxidized either in air or in water suggested by the dark color observed at the end. Therefore it is concluded that the crosslinking between Dopa groups took place revealing the mechanism for solidification.
For all cases, the coacervate adhesives perform better than popular commercial glue (α-cyanoacrylic resin adhesive) especially on aluminum substrates. Compared to other cases for the dry adhesion, the adhesion of coacervate (as shown in Table 1) was around 9-fold of the commonly Fe3+ incorporated adhesive13 and around 20-fold of mussel adhesive.29 Surprisingly, it is even much higher than white glue.22 In the case of wet adhesion, adhesives were cured underwater for 24 hours and then immediately measured after removing from water. The wet adhesion strength is about more than four times of the natural P. California adhesive.21,30,31
The performance is also much better than other metal-chelated Dopa based adhesives (as shown in Table 2).21,32 SEM result shown in Fig. 3c indicates the stretching of PAA-Dopa-Zn2+ coacervate after curing. Apparently, the material possesses certain cohesion forming the network and also exhibits adhesive property as expected.
| P. Californiaa | Dopa modified polymer-Fe3+b | Polyphospho-dopamide–Mg2+c | α-Cyanoacrylic resin adhesived | PAA-Dopa-Zn2+d |
|---|---|---|---|---|
| a Curing condition: the estimated bond strength of the natural P. California adhesive on glass.21b Curing condition: cured underwater for 24 h on aluminum substrates.32c Curing condition: cured on aluminum substrates underwater for 24 h at 37 °C.33d Curing condition: cured on aluminum substrates for 24 h at room temperature. | ||||
| 0.35 | 0.15 | 0.65 | 0.08 | 1.58 |
3.3 pH induced transformation from adhesive to hydrogels
Interestingly, a further increase of pH to 9 favors the formation of a hydrogel. These results are consistent with the iron mediated Dopa system reported previously.6,26,34 When pH was greatly increased, Dopa groups were oxidized accompanied by the appearance of dark color. The ionization of hydroxyl group enhances the chelating capability to Zn and therefore enables the crosslinking between zinc and two Dopa moieties.5 The only difference is that the weak coordination effects of zinc–Dopa system need a higher pH for gelation even though the zinc amount added is much greater than that of iron system because of the weaker chelation force of zinc. On the other hand, the electrostatic interaction between Zn and PAA can't be excluded at higher pH condition, but the simple addition of PAA into zinc solution does not generate a gel structure (just turbid solution) suggesting the gelation is mainly induced by the coordination between zinc and Dopa groups. Compared to the coacervate adhesives studied above, the elastic modulus (G′) of the hydrogel, as presented in Fig. 4, is larger than the viscous modulus (G′′) displaying a gel structure. The higher mechanical property of the gel structure is obtained by the irreversible oxidation of catechol group at higher pH which means the adjusting to less adhesive and more cohesive structure.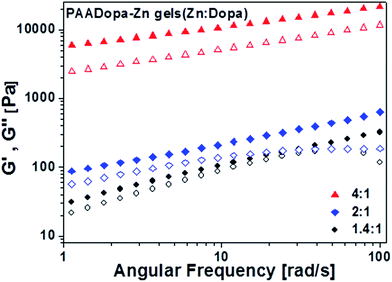 | ||
Fig. 4 Hydrogel formed after pH jump to pH 9. Frequency sweep at different Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dopa ratios (storage G′ and loss G′′ modulus, closed and open symbols, respectively). Dopa ratios (storage G′ and loss G′′ modulus, closed and open symbols, respectively). | ||
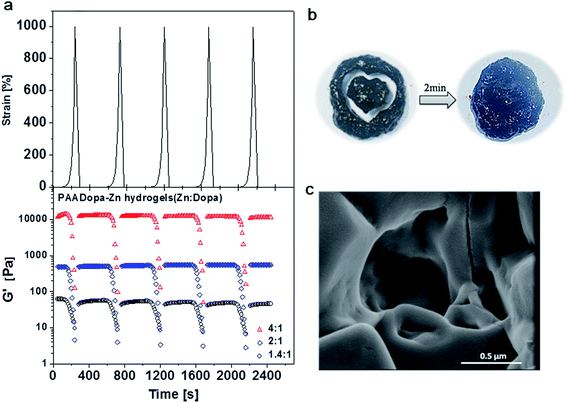
The gel also exhibits excellent self-healing properties. With rheometry straining the hydrogel until failure and afterward monitoring the recovery by small amplitude oscillation, the elastic moduli shown in Fig. 5a reveals the excellent restoration performance regardless of zinc content. The hydrogel with highest crosslinking degree recovered in less than 200 s to a high storage modulus (G′) of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Pa which is close to the initial value of 13
600 Pa which is close to the initial value of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 Pa therefore confirming that the reversible coordinate crosslinking nature of the complex catechol–Zn2+ bonds imparts this robust material with self-healing properties. Such quick recovery performance shows comparable or even better performance to Fe-gels.26,27
100 Pa therefore confirming that the reversible coordinate crosslinking nature of the complex catechol–Zn2+ bonds imparts this robust material with self-healing properties. Such quick recovery performance shows comparable or even better performance to Fe-gels.26,27
The self-healing ability was displayed in visual test by cleaving the hydrogel with a heart-shape mode (Fig. 5b). After the recovery of the external structure in two minutes, pulling and lifting the gel confirmed the recovered mechanical strength. SEM image from Fig. 5c also indicates the porous structure in contrast to the adhesive structure mentioned in Fig. 3c. Furthermore, the incorporated Zn2+ ions can be removed by aqueous solution containing agent such as ethylene diamine tetraacetic acid (EDTA) as mentioned in Fe3+ chelating system26 which indicates the metal-chelating plays a significant role in the present system (Fig. S6, ESI†).
4. Conclusions
A pH triggered zinc mediated coacervate was achieved based on Dopa-grafted PAA. It shows extraordinary adhesive performance under both dry and wet conditions. The maximum bonding strength on aluminum in the wet state can reach 1.5 M Pa. This study is superior to previous reports in terms of adhesion strength in both dry and wet states since metal ions simply crosslink polymers forming self-healing hydrogels. The mechanism of the formation of the extra coacervate state is proposed to involve the zinc chelated mono-catechol group electrostatically interacting with negative charged carboxylic groups from PAA chains and leading to a quick generation of the dense fluidic coacervates. In addition, the coacervate undergoes gelation by increasing pH, hence providing an ideal material for tissue adhesion with simultaneous therapeutical drug delivery at the adhesion point.Acknowledgements
Yisheng Xu and Xuhong Guo acknowledge the financial support from the National Natural Science Foundation of China (No. 51273063, 21476143, and 21306049), the Fundamental Research Funds for the Central Universities, the higher school specialized research fund for the doctoral program (222201313005 and 222201314029), 111 Project Grant (B08021), the Open Project of State Key Laboratory of Chemical Engineering (SKL-ChE-14C01) and Firmenich. Weina Wang acknowledges the China Scholarship Council.References
- J. H. Waite, N. H. Andersen, S. Jewhurst and C. Sun, J. Adhes., 2005, 81, 297–317 CrossRef CAS PubMed.
- M. Yu, J. Hwang and T. J. Deming, J. Am. Chem. Soc., 1999, 121, 5825–5826 CrossRef CAS.
- C. Fant, K. Sott, H. Elwing and F. Hook, Biofouling, 2000, 16, 119–132 CrossRef CAS PubMed.
- P. A. Suci and G. G. Geesey, J. Colloid Interface Sci., 2000, 230, 340–348 CrossRef CAS PubMed.
- B. K. Ahn, D. W. Lee, J. N. Israelachvili and J. H. Waite, Nat. Mater., 2014, 13, 867–872 CrossRef CAS PubMed.
- B. J. Kim, D. X. Oh, S. Kim, J. H. Seo, D. S. Hwang, A. Masic, D. K. Han and H. J. Cha, Biomacromolecules, 2014, 15, 1579–1585 CrossRef CAS PubMed.
- D. G. Barrett, D. E. Fullenkamp, L. He, N. Holten-Andersen, K. Y. C. Lee and P. B. Messersmith, Adv. Funct. Mater., 2013, 23, 1111–1119 CrossRef CAS PubMed.
- D. G. Barrett, G. G. Bushnell and P. B. Messersmith, Adv. Healthcare Mater., 2013, 2, 745–755 CrossRef CAS PubMed.
- H. J. Meredith, C. L. Jenkins and J. J. Wilker, Adv. Funct. Mater., 2014, 24, 3259–3267 CrossRef CAS PubMed.
- H. Lee, Y. Lee, A. R. Statz, J. Rho, T. G. Park and P. B. Messersmith, Adv. Mater., 2008, 20, 1619–1623 CrossRef CAS PubMed.
- J. J. Wilker, Nat. Chem. Biol., 2011, 7, 579–580 CrossRef CAS PubMed.
- H. T. Peng and P. N. Shek, Expert Rev. Med. Devices, 2010, 7, 639–659 CrossRef CAS PubMed.
- G. Westwood, T. N. Horton and J. J. Wilker, Macromolecules, 2007, 40, 3960–3964 CrossRef CAS.
- M. Palencia, B. L. Rivas, E. Pereira, A. Hernández and P. Prádanos, J. Membr. Sci., 2009, 336, 128–139 CrossRef CAS PubMed.
- T. L. Coombs and P. J. Keller, Aquat. Toxicol., 1981, 1, 291–300 CrossRef CAS.
- E. A. Wilkinson and C. I. Hawke, Cochrane Database of Systematic Reviews, 1998 DOI:10.1002/14651858.CD001273.
- D. J. Ennigrou, M. Ben Sik Ali and M. Dhahbi, Desalination, 2014, 343, 82–87 CrossRef PubMed.
- X. Guo, A. A. Abdala, B. L. May, S. F. Lincoln, S. A. Khan and R. K. Prud'Homme, Polymer, 2006, 47, 2976–2983 CrossRef CAS PubMed.
- P. Glass, H. Chung, N. R. Washburn and M. Sitti, Langmuir, 2009, 25, 6607–6612 CrossRef CAS PubMed.
- H. Lee, B. P. Lee and P. B. Messersmith, Nature, 2007, 448, 338–341 CrossRef CAS PubMed.
- H. Shao and R. J. Stewart, Adv. Mater., 2010, 22, 729–733 CrossRef CAS PubMed.
- C. R. Matos-Pérez, J. D. White and J. J. Wilker, J. Am. Chem. Soc., 2012, 134, 9498–9505 CrossRef PubMed.
- J. D. White and J. J. Wilker, Macromolecules, 2011, 44, 5085–5088 CrossRef CAS.
- B. P. Lee, J. L. Dalsin and P. B. Messersmith, Biomacromolecules, 2002, 3, 1038–1047 CrossRef CAS PubMed.
- N. Bandara, H. Zeng and J. Wu, J. Adhes. Sci. Technol., 2012, 27, 2139–2162 CrossRef PubMed.
- M. Krogsgaard, M. A. Behrens, J. S. Pedersen and H. Birkedal, Biomacromolecules, 2013, 14, 297–301 CrossRef CAS PubMed.
- N. Holten-Andersen, M. J. Harrington, H. Birkedal, B. P. Lee, P. B. Messersmith, K. Y. C. Lee and J. H. P. Waite, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2651–2655 CrossRef CAS PubMed.
- X. Guo, F. Deng, L. Li and R. K. Prud Homme, Biomacromolecules, 2008, 9, 1637–1642 CrossRef CAS PubMed.
- J. R. Burkett, J. L. Wojtas, J. L. Cloud and J. J. J. Wilker, J. Adhes., 2009, 85, 601–615 CrossRef CAS PubMed.
- H. Shao, K. N. Bachus and R. J. Stewart, Macromol. Biosci., 2009, 9, 464–471 CrossRef CAS PubMed.
- R. J. Stewart, C. S. Wang and H. Shao, Adv. Colloid Interface Sci., 2011, 167, 85–93 CrossRef CAS PubMed.
- J. Nishida, M. Kobayashi and A. Takahara, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1058–1065 CrossRef CAS PubMed.
- C. Sun, G. E. Fantner, J. Adams, P. K. Hansma and J. H. Waite, J. Exp. Biol., 2007, 210, 1481–1488 CrossRef CAS PubMed.
- M. S. Menyo, C. J. Hawker and J. H. Waite, Soft Matter, 2013, 9, 10314–10323 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: FT-IR and 1H-NMR spectra of PAA-Dopa. UV-vis detection of Dopa content. Zeta potential of PAA and PAA-Dopa at different pHs. Preparation of lap-shear test and the example of original data. EDTA treatment of zinc cross-linked PAA-Dopa hydrogel. See DOI: 10.1039/c5ra11915d |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |