Free-standing membranes made of activated boron nitride for efficient water cleaning†
Jie Li,
Huichao Jia,
Jing Lin,
Han Luo,
Zhenya Liu,
Xuewen Xu,
Yang Huang,
Peng Jin,
Jun Zhang,
Saleem Abbas and
Chengchun Tang*
School of Materials Science and Engineering, Hebei Key Laboratory of Boron Nitride Micro and Nano Materials, Hebei University of Technology, Tianjin 300130, P. R. China. E-mail: tangcc@hebut.edu.cn; Fax: +86-22-60202660; Tel: +86-22-60202660
First published on 18th August 2015
Abstract
Developing membranes with excellent mechanical strength and chemical stability is a practically important issue for efficient removal of pollutants from wastewater. In this work, we report on a free-standing membrane fabrication from an activated boron nitride (ABN) micro-ribbon. The membrane techniques we used, combine the intrinsic active adsorption competence of ABN and the mechanical advantages of conventional membrane filtration. The obtained membranes show an excellent removal ability of water pollutants through a simple filtration adsorption process. The examined pollutants include toxic metallic ions and organics. We showed that the dye (like methylene blue) removal ability significantly exceeded that of activated carbon by an order of magnitude at least; lead ions (Pb2+) in wastewater can be nearly fully removed, the starting 5 mg L−1 concentration was reduced to less than 0.01 mg L−1 after the 600 μm-thickness membrane adsorption filtration. Moreover, the membranes can be stacked together to further improve the adsorption capacity because of their high permeability. The excellent reusable performance of the filtration membranes was also confirmed. We believe that the reported work should open the way toward the practical application of ABN membranes in the field of wastewater purification.
1. Introduction
Ensuring reliable access to clean and affordable water is one of the greatest global challenges of this century.1 Worldwide, 884 million people lack adequate potable water and 1.8 million children die every year from diarrhea mainly due to water contamination.2 Water pollution removal has become more complex and difficult because of population growth and industrial development, so the magnitude of this challenge is rapidly increasing.3 Effective removal of pollutants from the environment by taking advantage of new materials and techniques has always been a significant subject. Among the various approaches available for wastewater purification, adsorption has been proven to be an effective technique due to its low-cost, high efficiency, and easy availability.4–9 Various adsorbents have been developed, mainly including activated carbon (AC), bioadsorbents and natural materials.10–13 However, the low adsorption or uptake gravimetric capacity, slow adsorption rate, and poor regeneration cycling ability hinder their further practical application in employing them as adsorption materials for water purification.Recently, aimed for the above mentioned drawbacks, hexagonal BN materials with porous structure have been developed to use as an effective adsorbent for the removal of dyes or toxic metal ions from water due to their high specific surface area, numerous density defects, low density, high stability in harsh conditions, and high resistance to oxidation and chemical inertness.7,14–19 Our group recently demonstrates that the activated boron nitride (ABN) exhibits the remarkably enhanced adsorption ability for various aromatic compounds and metal ions in water, as compared to the traditional activated carbon, in which more crystal defects and hydroxyl or organic surface groups have been introduced.20–22
Although great progresses have been made to improve adsorption performance for more widely-distributed pollutants, ABN in the form of powder tends to aggregate and consequently decreases the active surface sites during the practical adsorption applications. In addition, for regeneration it takes time and energy to separate adsorbents by filtration or high-speed centrifugation. Therefore, free-standing membranes with porous adsorbents as building blocks have been proposed in order to obtain a cost-effective and fast water purification performance,23–27 because the membrane adsorption is more economic and efficient than that of the powder.6,28–32 However, few literatures refer to the assembly of porous BN nanostructures into macroscopic membranes during water purification. Moreover the BN membranes could satisfy the requirement for practical and industrial applications, especially in harsh conditions.25
In this work, we propose a free-standing ABN membrane which combines the high capacity and ultrafast uptake characteristics of ABN and the ultrafiltration technique, by the hybridization of one-dimensional ABN micro-ribbon and 1D microfibrillated cellulose (MFC). Such technique which combines adsorption and filtration is a pressure-driven membrane process for separating dissolved molecules and toxic metal ions, which is faster and more efficient than adsorption alone. We demonstrate that the organics (methylene blue MB as an example) and metallic ions (like Pb2+) can be efficiently removed by using the novel membrane. The amount of MB adsorbed onto the hybrid membrane is nearly 4, 5 and 20 times higher than that of the pure ABN column, MFC membrane and AC column, respectively. The composite membrane also exhibits the excellent removal performance for the toxic ions. The high water flux and the stable regeneration efficiency were also verified. All these features make them attractive for practical applications in water purification systems.
2. Experimental
2.1. Synthesis of ABN and MFC
The preparation of ABN powder has been reported in our previous work.20 Typically, 3.71 g of boric acid, 3.78 g melamine, and 5 g P123 were mixed under vigorous stirring to get a homogenous solution with the pH of 6.5 adjusted by 0.1 M HNO3. After heating at 85 °C for 6 h, the white precipitate was washed with de-ionized water and dried at 90 °C for 12 h. ABN could be obtained after a two-step pyrolytic process: calcined at 546 °C for 2 h and at 1300 °C for 8 h in a flow of N2.The MFC slurry was prepared according to the procedure reported by Hu et al.33 200 mg of NaBr and 20 mL of NaClO aqueous solutions with an initial concentration of 12 wt% are added to the slurry (5 wt%, 200 mL) under gentle stirring. After reaction for 1 h, the fibrous product is obtained and thoroughly washed with distilled water.
2.2. Fabrication of ABN/MFC membrane
The free-standing ABN/MFC membranes were fabricated in a vacuum filtration process. The required ABN suspension was introduced drop by drop into the MFC solution diluted with the distilled water during stirring, followed by the addition of the surfactant Pluronic (F-127). The mixture was kept stirring for 60 min and placed in a bath by sonicating for 60 min to form homogeneous ABN/MFC suspension. Then 100 mL (1 wt%) of the suspension was poured into a Büchner funnel with inner diameter of 55 mm, in which a round polycarbonate membrane with a pore size of 200 nm was loaded, to form ABN/MFC membrane by suction using an oil pump press machine (Model SHZ-D (III), Zhengzhou Boke Analysis Instrument Factory Co., Ltd). The ABN/MFC membrane was then pressed under 2 MPa at room temperature. After drying in an oven at 150 °C for 2 h, the free-standing ABN/MFC membrane was obtained.2.3. Membrane characterization
The structure and morphology of the samples were characterized by field emission scanning electron microscopy (SEM, HITACHI S-4800). The pH values of the solutions were measured using a pH meter (PHS-25, Hangzhou). The Brunauer–Emmett–Teller (BET) specific surface area was measured at 77 K on an Autosorb iQ-C TCD analyzer. The pore size distribution was obtained by using the Barrett–Joyner–Halenda (BJH) method. The tensile strength of the ABN/MFC membrane was tested with a dynamic mechanical analysis (DMA) machine (Q800) under tension film mode. Fourier transform infrared (FTIR) spectrum was recorded on a Nicolet 7100 spectrophotometer between 400 and 4000 cm−1. A double beam UV/Vis spectrophotometer (HITACHI, U-3900H) was used to determine the concentration of MB. Concentrations of metal ions were measured by high dispersion inductively coupled plasma emission spectroscopy (TELEDYNE-Leeman Labs, USA).2.4. Membrane filtration adsorption experiments
To acquire pollutant solution, MB, neutral red and Congo red with the different type of ion charges were dissolved in deionized water and then diluted to the required concentration before use. The pH values of initial solutions were 8.0 for MB, 7.0 for neutral red, and 7.0 for Congo red. The appropriate amount of Pb(NO3)2 was dissolved in deionized water to obtain the solution with the initial concentration of 5 mg L−1, then the pH value was adjusted to 6.0. The filtration adsorption experiments were conducted by a filtration Büchner funnel. A piece of the as-prepared membrane with a thickness of 0.6 mm, a diameter of 55 mm, and a given weight of ∼1 g was placed in the Büchner funnel. A silicone O-ring was applied to compress the membrane to prevent lateral leaking. For high flow rate (up to 40 mL min−1), the pressures ranging from 180 to 190 kPa were supplied to the feed side of the different membranes and columns. Prior to the adsorption experiment, deionized water was added to equilibrate the membrane filtration system. MB and Pb(II) solutions were directly filtered through the membrane at various constant flow rates, respectively. The effluent at each stage was collected and analyzed. For comparison, we measured the filtration adsorption performances of a pure MFC membrane, ABN column and AC column, respectively.2.5. Regeneration and reusability
Desorption solutions (1 M HCl in 50% methanol) were loaded at the same rate of the initial filtration adsorption to elute the as-absorbed cationic dye and Pb(II). Then the membrane was filtered and washed with deionized water to remove the residual HCl and methanol. To evaluate the membrane-regeneration capacity, the above adsorption cycles were repeated in ten times.3. Results and discussion
3.1. Characterization of ABN/MFC membranes
A schematic illustration of the ABN/MFC composite membrane configuration is displayed in Fig. 1a. In the composite membrane, 1D ABN ribbon is aligned in the planar direction, which can maximize the overlapping amount to increase active adsorption sites of the membrane. MFC fibers are used as a glue to enhance the mechanical strength and flexibility of the membrane by wrapping the ABN together.26 To enhance the adsorption rate and capacity of the composite membrane, the ABN content should be higher than that of MFC, while meeting the mechanical strength for filtration. Fig. 1b demonstrates the filtration adsorption process of MB solution through the filtrated membrane. As a result, the MB species are completely removed from the aqueous solution in the initial operation stage. | ||
| Fig. 1 (a) Schematic diagram of the ABN/MFC composite structure. (b) A schematic to show how MB species are removed by the membrane from aqueous solution. | ||
Highly activated BN were prepared by a simply two-step pyrolytic process with the presence of P123 as structure-directed agent, and exhibits a ribbon-like microstructure with extremely high surface area of 2078 m2 g−1, large pore volume of 1.66 cm3 g−1 and richer hydroxyl/organic groups such as B–OH/B–NH2.20,31 The MFC binding agent treated with NaBr/NaClO introduces plenty of oxygen-containing groups (–OH) onto its surface.33–35 Homogeneous ABN/MFC suspension was facilely obtained by mixing above two obtained species with distilled water under stirring. As shown in Fig. 2a, no agglomerates could be found and the MFC solution was thus compatible with the ABN solution, mainly because the repulsive forces generated by their surface functional groups provide stability in hybrid solution. Additionally, the introduction of surfactant Pluronic (F-127) also made the hybrid solution dispersion more uniform and stable.
ABN/MFC membranes were a flat paper-like material that could be detached easily from the substrate, as shown in Fig. 2b. We also found that the well dispersed mother solution is vital in obtaining the uniform composite membrane without any cracks or pinholes. The uniform membrane is a key factor to achieve excellent mechanical flexibility and strength. Filtration membrane of 0.6 mm thickness and 55 mm diameter with different ratios of ABN can be controlled precisely by varying the volume and concentration of the ABN and MFC solution. Herein, we fabricated three different hybrid membranes with different ABN contents, namely ABN/MFC-70, ABN/MFC-40 and ABN/MFC-10, in which the number represents the ABN weight ratios of the total mass of ABN and MFC. The field emission scanning electron microscopy (SEM) image in Fig. 2c reveals that the membrane consists of many randomly oriented 1D ABN and 1D MFC. Both two fibers interconnect and tightly pack together each other to form the high-porosity membrane.31,36,37
Because ABN/MFC membranes are self-supporting, their mechanical properties are critical in wastewater treatment for filtration adsorption. Fig. 3a is an optical image of the bent ABN/MFC composite membranes, indicating its excellent mechanical flexibility. We examined quantitatively the mechanical strength of the membranes as shown in Fig. 3b. Generally, the tensile strength reduces with an increase of the ABN loading content. It is worth noting that the tensile strength of pure ABN micro-ribbon film is too weak to behave a freestanding film, indicating the significance of incorporating MFC as the mechanical stabilizer due to its high strength of 220 MPa. The filter with 70 wt% ABN has a high tensile strength of 30 MPa, which is mechanically strong enough for filtration adsorption even under a fast flux.
 | ||
| Fig. 3 (a) ABN/MFC-70 with higher flexibility. (b) Tensile strength vs. ABN content in the composite membrane. | ||
To understand the effect of surface functional groups on the adsorption capacity, ion-exchange capacity (IEC) was measured by using an acid–base titration method,38 determining from the reduction in alkalinity. The commercial AC column was tested for comparison. The experimental data provided in Table 1 clearly illuminate that the IEC of as-obtained ABN/MFC samples is much higher than that of AC (0.44 mmol g−1), though the AC have a higher BET surface area, respectively. The higher IEC with relation to the abundant functional groups is believed to be the key point to the higher adsorption capacity than that of commercial AC. The IEC 8.7 mmol g−1 of ABN/MFC-70 is higher than those of ABN/MFC-40 and ABN/MFC-10, due to higher ABN ratio loading. Therefore, ABN/MFC-70 with abundant functional groups (–OH), which is identified by the FTIR spectrum (Fig. S1, ESI†), should have the best filtration adsorption performance of water-purification. This is because the abundant functional groups on both ABN and MFC not only guarantee the homogeneousness and stability of the mother ABN/MFC suspension, but also have great potential for contaminant removal by the adsorption process.
| Adsorbent samples | IEC [mmol g−1] | BET surface area [m2 g−1] |
|---|---|---|
| ABN/MFC-70 | 8.7 | 1106 |
| ABN/MFC-40 | 4.2 | 585 |
| ABN/MFC-10 | 2.3 | 217 |
| AC | 0.44 | 840 |
The water contact angle (CA) measurement for the ABN/MFC-70 shown in Fig. S2 (ESI†) has demonstrated that it exhibits well hydrophilic, which results in enhanced membrane flux. In addition, the CAs of the ABN/MFC-70, ABN/MFC-40, ABN/MFC-10 and pure MFC membrane were also summarized in Table S1.† For the ABN/MFC-70, two different sizes of Au nanoparticles were used to investigate its size distributions and size selectivity. About 91% of Au nanoparticles (diameter: 15 nm) could freely pass through the ABN/MFC-70. In contrast, 35 nm Au nanoparticles were almostly rejected from the solution, as shown in Fig. S3 (ESI†). This result indicates that the ABN/MFC-70 is ultrafiltration membrane and shows high size-selective rejection properties. Fig. S4 (ESI†) illustrates the nitrogen adsorption/desorption isotherm and the corresponding pore size distribution of the ABN/MFC-70. The measured isotherm (Fig. S4a†) can be classified as a type-II isotherm according to the International Union of Pure and Applied Chemistry nomenclature, and exhibit an H3 hysteresis loop. By using the BJH, the pore size distribution of the ABN/MFC-70 mainly ranges from 1.3 to 20 nm, as shown in Fig. S4b (ESI†).
3.2. Filtration adsorption and regeneration studies
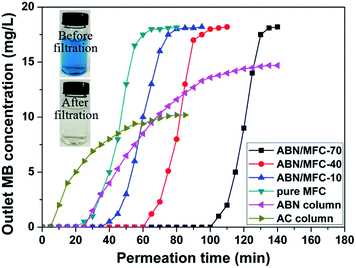
MB was selected as a model pollutant to assess the efficiency of the filtration membrane of ABN/MFC. Based on our previous work for MB adsorption,22 the pH value of the solution and the temperature were set at 8.0, and 30 °C, respectively. Fig. 4 displays the breakthrough curves for MB solution (20 mg L−1) through the different filtration membranes and columns of AC and ABN at a constant flow rate of 20 mL min−1. The time of the complete rejection of MB from the aqueous solution by the ABN/MFC-70 was 100 min (the inset showing the photos of MB solution before and after filtration adsorption), while 20 min of the pure MFC. Namely, the adsorption capacity of the ABN/MFC-70 is estimated 2400 mL at 80% breakthrough and 2200 mL at 10% breakthrough. This adsorption value is about 5 times higher than that of the pure MFC membrane. We also note that the time for the complete rejection of MB through the ABN filtration column was only 25 min, although the dosage of ABN in the column was 30% higher than that of ABN in the ABN/MFC-70. The slower adsorption ability and rate for the ABN column could be due to hindering adsorbate diffusion through the aggregated and closely stacked ABN. In particular, the solution-phase adsorption of aromatic compounds is mainly controlled by the available adsorption surface area rather than pore volumes.26 Additionally, the time of the AC filtration column was only 5 min, which was nearly one twentieth of ABN/MFC-70. The inset in Fig. 4 shows the photo of MB solution before and after filtration adsorption by using ABN/MFC-70.The molecular diameter (0.8 nm) of MB39 is much less than the pore size of the ABN/MFC membranes. A reasonable mechanism for the rejection of MB in the initial filtration stage is that the MB molecules are adsorbed onto the surface and pores of ABN/MFC membranes by electrostatic, complex, or hydrogen-bonding interactions. Therefore, the excellent water purification performance of ABN/MFC-70 may be attributed to abundant functional groups, high BET surface area, the effective intercalation and distribution of ABN in MFC, the more sites available for adsorption, and the formation of an interconnected network between the composite structures.40,41
Our recent work demonstrates that activated BN powder has a more negative charge surface due to the protonation of the hydroxyl and amino groups, resulting in more excellent MB and toxic metal ions removal abilities than other neutral and anionic dyes. Therefore, the present study indicates that the rich surface group characteristic of the ABN is still kept in the membrane structure even though the enough mechanical strength was rendered.
To further confirm the advantages of our membrane with excellent MB removal ability, we also measured the filtration adsorption performances for neutral dye (neutral red) and anionic dye (Congo red). The corresponding breakthrough curves of ABN/MFC-70 are shown in Fig. 5. The breakthrough time of neutral red solution is 30 min and 10 min for Congo red, which is about 0.33 and 0.10 times of the time of the MB rejection. Therefore, the strong electrostatic interaction between dye and ABN/MFC-70 is the key factor to control the adsorption and separation capacity, because the three dye molecules possess the completely different ionic feature.
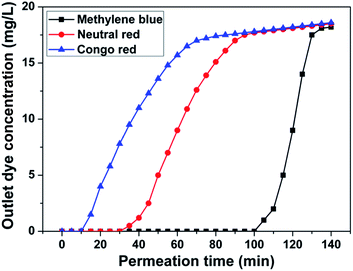 | ||
| Fig. 5 Comparison of breakthrough curves of methylene blue, neutral red, and Congo red through the ABN/MFC-70, respectively. | ||
The experiments of MB solutions passed through the ABN/MFC-70 with different fluxes were carried out to analyze the relationship between the breakthrough time and the filtration adsorption ability, as displayed in Fig. 6. Higher flux at 40 mL min−1 causes earlier breakthrough at ∼45 min. The higher flux brought more MB molecules onto the composite membrane surface at the certain time; the available adsorption sites for MB adsorption were quickly consumed, so that the time was decreased with the increasing pressure.42 Nevertheless, the MB species could be still completely removed by the filtration membrane from ∼1800 mL of solution even when a very high flux of 40 mL min−1 was used due to its very rapid adsorption and separation capacity. Therefore, our ABN/MFC membrane with a high filtration rate (or low pressure operation) should have a significant merit in practical use.
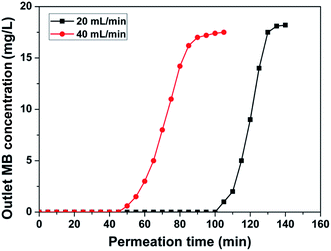 | ||
| Fig. 6 Breakthrough curves for MB solutions through the ABN/MFC-70 at two flow rates (20 mL min−1 and 40 mL min−1), at pH 8.0, and temperature 30 °C. | ||
To improve adsorption capacity for organic pollutants, many layers of filtration membranes can be stacked together to obtain a larger membrane area. Stacking membranes still maintain high water flux at a relatively low pressure because of high permeability of the ABN/MFC-70. Namely they can still keep on a low pressure drop ranging from 180 to 190 kPa even when the MB aqueous solution with a high constant flow rate of 20 mL min−1 go through the layer three membranes. In addition, it is noted that the permeability and filtration adsorption performance hardly decrease under the transmembrane pressures due to the high mechanical strength and flexibility of the ABN/MFC-70 (Fig. 3). The breakthrough curves of two and three layers of membranes for filtration of MB solution were tested respectively, as depicted in Fig. 7. The results reveal that the MB species were completely removed by the single membrane from over 2000 mL of the aqueous solution, which was almost one half and one third of the dosages of MB adsorption by a stack of two membranes and three membranes, respectively. Obviously, stacking does not damage the removal performance of individual membrane, thus offers a simple and effective method to improve the wastewater treatment capacity.
The recycle of filtration membrane is very important for practical applications. MB molecules are adsorbed on ABN/MFC possibly by complex chemical bonds, such as electrostatic, complex, or hydrogen-bonding interactions. The bonds can be easily destroyed by 1 M HCl in 50% methanol to remove MB from the membrane. Such simple regeneration experiment was carried out to estimate the cyclic property of filtration adsorption of a single ABN/MFC-70 for MB. The experimental results indicate that, as seen in Fig. 8, the filtration adsorption capacity at 10% breakthrough had not remarkable change even after ten complete runs. This suggests no obvious deteriorations in the MB-removal ability and mass losses of the filtration membrane as the membrane-filtration processes are executed in the successively adsorption–desorption washing runs.
 | ||
| Fig. 8 Breakthrough curves for adsorption of MB by the fresh ABN/MFC-70 and the regenerated membrane after ten adsorption–desorption washing cycles. | ||
To further estimate the filtration adsorption characteristics of ABN/MFC-70, we also measured the extraction of toxic pollutant ions from wastewater. The lead ions (Pb2+) were selected as the model metallic pollutant ions, which is one of the most toxic elements to human beings. Fig. 9a shows the breakthrough curves for Pb(II) solution (5 mg L−1) through ABN/MFC-70 and AC column at a constant flow rate of 2.0 mL min−1, respectively. The experimental data illustrate that the ABN/MFC-70 could remove Pb(II) almost completely from solution (the resulting Pb2+ concentration being less 0.01 mg L−1) of over 2000 mL, which corresponds to about 17 h of operation time under the same experimental conditions. Whereas, the Pb(II) concentration of first 10 mL effluent from the AC column was above 1 mg L−1, although the mass of AC in the column was the same as that of ABN/MFC. The removal capacity of Pb(II) onto ABN/MFC-70 is much higher than that of most of the membranes.43–46 In addition, the cycle properties of ABN/MFC-70 can be estimated via a simple adsorption and desorption test, as shown in Fig. 9b. The result reveals that, even after ten filtration adsorption and regeneration cycles, ABN/MFC-70 could remove Pb(II) almost completely from the solution of over 1100 mL. Namely, the removal efficiency of over 50% was retained in 10 consecutive cycles, which is consistent with the previous work.21 The effective removal and regeneration suggest that the composite membranes could capture pollutants such as metallic cations (Pb(II)), cationic dye (MB) with positive charges by similar means of filtration adsorption mechanism. These simple comparisons further exemplify the advantages of ABN membranes for the water purification.
4. Conclusions
In summary, we have fabricated the ABN membrane based on the combination of excellent adsorption performance of ABN, unique flow properties of fibrous membrane, and in-plane coupling between 1D BN micro-ribbon and MFC. The resulting ABN/MFC membranes exhibit fast and efficient water purification ability as well as mechanically robust enough to be used for practical use. Substantial adsorption experiments were conducted to demonstrate the absorption rate and capacity of both organic dyes and toxic metal ions onto the composite membrane. By using the 70 wt% of ABN, the adsorption ability of the membrane significantly exceeded that of activated carbon by an order of magnitude at least. The high regeneration efficiency also has been demonstrated. All the features render the ABN membrane a promising filter to obtain clean and fresh drinking water, or a new component of existing filtration systems to water purification.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51332005, 51372066, 51172060, 51202055, 21103056, 51402086), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT: IRT13060), the Natural Science Foundation of Hebei Province (Grant No. E2012202040), and the Innovation Fund for Excellent Youth of Hebei University of Technology (No. 2012001).Notes and references
- T. Tao and X. Kunlun, Nature, 2014, 511, 527–528 CrossRef CAS PubMed.
- X. Qu, J. Brame, Q. Li and P. J. J. Alvarez, Acc. Chem. Res., 2012, 46, 834–843 CrossRef PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- J. Li, J. Lin, X. Xu, X. Zhang, Y. Xue, J. Mi, Z. Mo, Y. Fan, L. Hu, X. Yang, J. Zhang, F. Meng, S. Yuan and C. Tang, Nanotechnology, 2013, 24, 155603 CrossRef PubMed.
- I. Ali, Chem. Rev., 2012, 112, 5073–5091 CrossRef CAS PubMed.
- H.-W. Liang, X. Cao, W.-J. Zhang, H.-T. Lin, F. Zhou, L.-F. Chen and S.-H. Yu, Adv. Funct. Mater., 2011, 21, 3851–3858 CrossRef CAS PubMed.
- W. Lei, D. Portehault, D. Liu, S. Qin and Y. Chen, Nat. Commun., 2013, 4, 1777 CrossRef PubMed.
- R. Demir-Cakan, N. Baccile, M. Antonietti and M.-M. Titirici, Chem. Mater., 2009, 21, 484–490 CrossRef CAS.
- Y.-J. Xu, G. Weinberg, X. Liu, O. Timpe, R. Schlögl and D. S. Su, Adv. Funct. Mater., 2008, 18, 3613–3619 CrossRef CAS PubMed.
- G. Crini, Bioresour. Technol., 2006, 97, 1061–1085 CrossRef CAS PubMed.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70–80 CrossRef CAS PubMed.
- T. K. Naiya, A. K. Bhattacharya and S. K. Das, J. Colloid Interface Sci., 2009, 333, 14–26 CrossRef CAS PubMed.
- G. Bereket, A. Z. Arog and M. Z. Özel, J. Colloid Interface Sci., 1997, 187, 338–343 CrossRef CAS.
- J. A. Perdigon-Melon, A. Auroux, C. Guimon and B. Bonnetot, J. Solid State Chem., 2004, 177, 609–615 CrossRef CAS PubMed.
- Q. Weng, X. Wang, C. Zhi, Y. Bando and D. Golberg, ACS Nano, 2013, 7, 1558–1565 CrossRef CAS PubMed.
- R. T. Paine and C. K. Narula, Chem. Rev., 1990, 90, 73–91 CrossRef CAS.
- C. Zhi, Y. Bando, C. Tang, H. Kuwahara and D. Golberg, Adv. Mater., 2009, 21, 2889–2893 CrossRef CAS PubMed.
- G. Lian, X. Zhang, S. Zhang, D. Liu, D. Cui and Q. Wang, Energy Environ. Sci., 2012, 5, 7072–7080 CAS.
- F. Liu, J. Yu, X. Ji and M. Qian, ACS Appl. Mater. Interfaces, 2015, 7, 1824–1832 CAS.
- J. Li, X. Xiao, X. Xu, J. Lin, Y. Huang, Y. Xue, P. Jin, J. Zou and C. Tang, Sci. Rep., 2013, 3, 3208 CrossRef PubMed.
- J. Li, P. Jin and C. Tang, RSC Adv., 2014, 4, 14815–14821 RSC.
- J. Li, Y. Huang, Z. Liu, J. Zhang, X. Liu, H. Luo, Y. Ma, X. Xu, Y. Lu, J. Lin, J. Zou and C. Tang, J. Mater. Chem. A, 2015, 3, 8185–8193 CAS.
- K. C. Krogman, J. L. Lowery, N. S. Zacharia, G. C. Rutledge and P. T. Hammond, Nat. Mater., 2009, 8, 512–518 CrossRef CAS PubMed.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. Suib, L. J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332–336 CrossRef CAS PubMed.
- G. Lian, X. Zhang, H. Si, J. Wang, D. Cui and Q. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 12773–12778 CAS.
- A. B. Dichiara, T. J. Sherwood, J. Benton-Smith, J. C. Wilson, S. J. Weinstein and R. E. Rogers, Nanoscale, 2014, 6, 6322–6327 RSC.
- H.-W. Liang, L. Wang, P.-Y. Chen, H.-T. Lin, L.-F. Chen, D. He and S.-H. Yu, Adv. Mater., 2010, 22, 4691–4695 CrossRef CAS PubMed.
- P. Vandezande, L. E. M. Gevers and I. F. J. Vankelecom, Chem. Soc. Rev., 2008, 37, 365–405 RSC.
- J. Kim and B. van der Bruggen, Environ. Pollut., 2010, 158, 2335–2349 CrossRef CAS PubMed.
- R. N. Muthu, S. Rajashabala and R. Kannan, Int. J. Hydrogen Energy, 2015, 40, 1836–1845 CrossRef PubMed.
- H. Zhu, Y. Li, Z. Fang, J. Xu, F. Cao, J. Wan, C. Preston, B. Yang and L. Hu, ACS Nano, 2014, 8, 3606–3613 CrossRef CAS PubMed.
- X. Li, J. Li, J. Wang, H. Wang, C. Cui, B. He and H. Zhang, J. Membr. Sci., 2014, 451, 226–233 CrossRef CAS PubMed.
- H. Zhu, Z. Xiao, D. Liu, Y. Li, N. J. Weadock, Z. Fang, J. Huang and L. Hu, Energy Environ. Sci., 2013, 6, 2105–2111 CAS.
- I. Siró and D. Plackett, Cellulose, 2010, 17, 459–494 CrossRef.
- J. Huang, H. Zhu, Y. Chen, C. Preston, K. Rohrbach, J. Cumings and L. Hu, ACS Nano, 2013, 7, 2106–2113 CrossRef CAS PubMed.
- S. Maphutha, K. Moothi, M. Meyyappan and S. E. Iyuke, Sci. Rep., 2013, 3, 1509 Search PubMed.
- X. Li, J. Li, J. Wang, H. Wang, B. He, H. Zhang, W. Guo and H. H. Ngo, J. Membr. Sci., 2014, 453, 18–26 CrossRef CAS PubMed.
- G.-J. Hwang, H. Ohya and T. Nagai, J. Membr. Sci., 1999, 156, 61–65 CrossRef CAS.
- E. N. E. Qada, S. J. Allen and G. M. Walker, Chem. Eng. J., 2008, 135, 174–184 CrossRef PubMed.
- G. Dongtao, W. Dewang, S. Wei, M. Yuanyuan, T. Xiangdong, L. Pengfei and Z. Qiqing, Biomed. Mater., 2006, 1, 170 CrossRef PubMed.
- J. Yeo, S. Y. Kim, S. Kim, D. Y. Ryu, T. H. Kim and M. J. Park, Nanotechnology, 2012, 23, 245703 CrossRef CAS PubMed.
- L. Zheng, Y. Su, L. Wang and Z. Jiang, Sep. Purif. Technol., 2009, 68, 244–249 CrossRef CAS PubMed.
- C.-V. Gherasim, G. Bourceanu and D. Timpu, Chem. Eng. J., 2011, 172, 817–827 CrossRef CAS PubMed.
- R. Yang, K. B. Aubrecht, H. Ma, R. Wang, R. B. Grubbs, B. S. Hsiao and B. Chu, Polymer, 2014, 55, 1167–1176 CrossRef CAS PubMed.
- Z. Reddad, C. Gérente, Y. Andrès, J.-F. Thibault and P. le Cloirec, Water Res., 2003, 37, 3983–3991 CrossRef CAS.
- C.-V. Gherasim, J. Cuhorka and P. Mikulášek, J. Membr. Sci., 2013, 436, 132–144 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11899a |
| This journal is © The Royal Society of Chemistry 2015 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: