Bioinspired preparation of monolithic ordered mesoporous silica for enrichment of endogenous peptides†
Gang-Tian Zhuab,
Xi Chenc,
Xiao-Mei Hea,
Zheng Zhanga,
Xiao-Shui Lid,
Bi-Feng Yuana and
Yu-Qi Feng*a
aKey Laboratory of Analytical Chemistry for Biology and Medicine (Ministry of Education), Department of Chemistry, Wuhan University, Wuhan 430072, P.R. China. E-mail: yqfeng@whu.edu.cn; Fax: +86-27-68755595; Tel: +86-27-68755595
bKey Laboratory of Tectonics and Petroleum Resources (Ministry of Education), China University of Geosciences, Wuhan 430074, China
cWuhan Institute of Biotechnology, Wuhan 430072, China
dState Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences, Wuhan 430074, China
First published on 1st September 2015
Abstract
In the current study, monolithic ordered mesoporous silica (MOMS) was successfully prepared by a modified Stöber method using natural pomelo peel and cetyltrimethylammonium bromide (CTAB) as dual templates. A mesostructured CTAB/silica composite was deposited on the carbohydrate-based surface of the sponge-like pomelo peel. After removing the templates by calcination and acid treatment, MOMS with a continuous skeleton, interconnecting macropores and ultra-high surface area (1120 m2 g−1) was obtained. MOMS materials with various and controllable sizes and shapes could be prepared simultaneously in a one-pot synthesis. Small pieces of MOMS (millimeter size) were then applied as the adsorbent to enrich peptides. Owing to the ordered mesoporous structure, the as-prepared MOMS was demonstrated to be an excellent adsorbent for effective enrichment of endogenous peptides from human plasma.
1. Introduction
Because of the uniform pore size, high surface area and large pore volume, ordered mesoporous silica (OMS) has great utility in separation sciences, catalysis, sensors, drug delivery and nanocasting.1–6 Since its discovery, OMS has drawn wide interest and OMS materials with various porous structures were prepared.7,8 However, OMS materials with nano or submicro-meter size are not suitable to serve as packed adsorbents or separation media though they exhibit fast equilibrium in molecule transportation.9 Enlargement of the size of the OMS may be a solution to the problem,10,11 but the long molecule transportation distance from the deep mesopore of these particles could cause slow equilibrium in practical applications. In this respect, preparation of millimeter-sized OMS without production of long mesopores will benefit the applications of OMS in the separation sciences.Monolithic ordered mesoporous silica (MOMS) is an interesting material that combines the advantages of mesopores and macropores, in addition to its macroscopic appearance.12–14 Mesopores contribute high surface area for the material and size-selectivity for target molecules. In addition, macropores can provide excellent performance of mass transport and offer easier accessibility for analytes.15,16 On the other hand, monolithic pieces can be advantageous for the porous materials that are employed as electrode, catalytic microreactor, or packing material for adsorption and separation.17,18 Hitherto, impregnation with dual templates has been developed for synthesis of MOMS materials.19,20 For instance, MOMS was prepared with dual templates of surfactant and hard colloidal crystal.21,22 The product showed uniform macropores and primary framework with nanoscale to sub-microscale dimensions. However, the mesostructure showed relatively low periodicity. In addition, the preparation is time-consuming and labor-intensive, and it needs special care against excess gel precursor. MOMS could also be prepared without hard template.23–25 But the drawback of this method is the requirement to simultaneously optimize the skeleton morphology and mesostructure, which may lead to heterogeneous skeleton26 or mesopores.27
Recently, bioinspired synthesis using renewable biomaterials as templates have drawn much attention. Cellulose aerogel,28 natural cellulose substance29,30 and filter papers31 have been used as hard templates in preparation of functional silica materials. However, their application as templates for synthesis of MOMS has seldom been reported. Pomelo peel is a sponge-like natural product composed of cellulose and pectin as major constituents.32,33 With the natural three-dimensional structure and convenient tuning of size and shape, pomelo peel should be a suitable hard template for the preparation of MOMS.
In the current work, MOMS with macropore windows and ordered mesopores was successfully prepared by a simple Stöber method using pomelo peel as the hard template and CTAB as the soft template. The prepared MOMS showed bulk appearance, macroporous network, ordered mesostructure, ultra-high surface area (1120 m2 g−1) and large pore volume (0.91 cm3 g−1). Different from most previous work,22,34,35 modified Stöber method was used to prepare MOMS, and various and controllable sizes and shapes of MOMS can be large-scale prepared simultaneously in one-pot synthesis. Using pomelo peel fragments minced by a juicer as hard template, the prepared MOMS was further applied to effective enrichment of endogenous peptides.
2. Experimental section
2.1 Chemicals and materials
Cetyltrimethylammonium bromide (CTAB), ethanol, ammonia hydrate (NH3·H2O, 25 wt% in H2O), urea, calcium chloride (CaCl2) and hydrochloric acid (HCl, 37 wt% in H2O) were purchased from Shanghai General Chemical Reagent Factory (Shanghai, China). Tetraethyl orthosilicate (TEOS) was obtained from the Chemical Plant of Wuhan University (Wuhan, China). HPLC grade acetonitrile (ACN) was obtained from Fisher Scientific (Pittsburgh, USA). Cotton wool was supplied by Xuzhou Hygiene of Material Factory (Xuzhou, China). α-Cyano-4-hydroxycinnamic acid (CHCA), trifluoroacetic acid (TFA), lysozyme, horseradish peroxidase (HRP) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St Louis, USA). Sequencing grade trypsin was obtained from Promega (Madison, WI, USA). Human plasma sample was obtained from the Wuhan Zhongnan Hospital according to the standard clinical procedures and stored at −80 °C until use. Purified water was obtained with a Milli-Q apparatus (Millipore, Bedford, MA, USA). Pomelos were purchased at a local market. Pomelo peels were cut into centimeter-sized pieces with special shapes or minced by a juicer to obtain small pieces (millimeter-size). The pomelo peel pieces were first treated with ethanol/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) containing 2.5% NH3·H2O at room temperature for 6 h to clean the compositions of pomelo peel that may dissolve in the Stöber solution and then rinsed repeated with deionized water and ethanol. The pomelo peel pieces were finally dried and stored at a sealing bag till use.
1, v/v) containing 2.5% NH3·H2O at room temperature for 6 h to clean the compositions of pomelo peel that may dissolve in the Stöber solution and then rinsed repeated with deionized water and ethanol. The pomelo peel pieces were finally dried and stored at a sealing bag till use.
2.2 Preparation of MOMS
The synthesis procedure is partly adapted from the Stöber method for mesoporous silica spheres.36,37 Typically, 1.5 g of pomelo peel pieces was added into a mixture of ethanol (200 mL), deionized water (200 mL), CTAB (4 g) and NH3·H2O solution (5 mL). After stirring for 30 min at room temperature, 5.0 mL of tetraethyl orthosilicate (TEOS) was slowly added to the solution with continuous stirring. After 6 h of reaction, pomelo peel/CTAB/SiO2 composite was collected with filtration using a strainer (hole size: 1 mm) by gravity and washed repeatedly with deionized water and ethanol to remove the nanoscale or microscale by-product. Then the composite was calcined at 550 °C for 6 h to remove CTAB and the most components of pomelo peel. Finally, the product was treated with 6 M HCl solution for 0.5 h to remove metal salts derived from pomelo peel, and then the pure MOMS was obtained.2.3 Material characterization
The composition of materials was determined by Shimadzu EDX-720 energy-dispersive X-ray analysis (EDX, Kyoto, Japan) using Mg Kα radiation as the excitation source. The microscopic morphology of materials was examined by a Quanta 200 scanning electron microscopy (SEM) (FEI, Holland). Transmission electron microscopy images were obtained from JEM-2100 (HT) transmission electron microscope (TEM, JEOL, Japan). The powder X-ray diffraction (XRD) measurement was recorded on a D/MAX-RB X-ray powder diffractometer (RIGAKU, Japan) using Cu Kα radiation (λ = 1.5406 Å) with scattering angles (2θ) of 1–8°. Nitrogen sorption measurement was performed at 77 K using a JW-BK surface area and pore size analyzer (JWGB Sci. & Tech., Beijing, China). The composites were activated by evacuating in vacuum and heating to 423 K for 6 h to remove any physically adsorbed substances before analysis. The specific surface area value was calculated according to the BET (Brunauer–Emmett–Teller) equation at P/P0 between 0.05 and 0.3. The pore parameters (pore volume and pore diameter) were evaluated from the desorption branch of isotherm based on BJH (Barrett–Joyner–Halenda) model. The macroporous property of the MOMS was determined by mercury intrusion porosimetry using an Autopore IV 9500 mercury porosimeter (Micromeritics, Norcross, USA).2.4 Preparation of MOMS packed syringe
As shown in Fig. S1,† small pieces (1–3 mm) of MOMS were packed in the hub of a 1 mL syringe.38 To ensure the hub was fully filled and avoid the MOMS moving during the extraction process, the MOMS was packed between two cotton layers. Typically, a small piece of cotton wool (approximate 200 μg) was pushed into the hub. Then MOMS (1 mg) was added followed by covering with another piece of cotton wool (approximate 100 μg). The sandwich packing bed was compacted tightly when the barrel was connected to the hub.2.5 Preparation of peptides
BSA (1 mg) was dissolved in 100 μL of denaturing buffer solution (8 M urea in 100 mM Tris–HCl, pH 8.5). The obtained protein solution was mixed with 5 μL of 100 mM tri(2-chloroethyl)phosphate (TCEP) and incubated for 20 min at 37 °C to reduce protein disulfide bonding. Then 3 μL of 500 mM iodoacetamide was added, and the resulting solution was incubated for an additional 30 min at 37 °C in the dark. The reduced and alkylated protein mixture was diluted with 300 μL of 100 mM Tris–HCl (pH 8.5). Subsequently, 9 μL of 100 mM CaCl2 was added, and the mixture was digested with trypsin at an enzyme to substrate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (w/w) by incubating overnight at 37 °C.
50 (w/w) by incubating overnight at 37 °C.
2.6 Peptide enrichment with MOMS using the lab-in-syringe SPE approach
The MOMS-packed syringe was directly used for peptide enrichment. BSA digests or standard protein aqueous solution (500 μL) was pipetted up and down 20 times to allow peptides adsorb on the MOMS. After washing twice with 500 μL water, the trapped peptides were eluted with 50 μL of 50% ACN containing 0.1% TFA. Finally, original solution (1 μL), solution after extraction (1 μL) and eluate (1 μL) were directly applied for MALDI-TOF MS analysis.Human plasma (50 μL) was diluted 10 times with water and spiked with 20 nM synthetic peptide (ERVYIHPF, m/z 1060.55), and the solution was pipetted up and down 30 times. Then the packed bed was successively washed with water (500 μL) three times. The adsorbed peptides were eluted with 50 μL of 50% ACN containing 0.1% TFA and 1 μL of the eluate was applied for MALDI-TOF MS analysis. When the peptide enrichment was applied for LC-MS/MS analysis, no standard peptide was added into the human plasma sample.
2.7 Peptide enrichment with OMS microspheres
For comparison, OMS microspheres was prepared and used to enrich peptides from BSA digests.39,40 Briefly, 1 mg of OMS microspheres was dispersed in 500 μL of 20 nM BSA digests in aqueous solution. After incubated at 37 °C for 15 min, the supernatant was removed after centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 3 min. After washing twice with water, the trapped peptides were eluted with 50 μL of 50% ACN containing 0.1% TFA. Finally, 1 μL of the eluate was directly applied for MALDI-TOF MS analysis.
000 g for 3 min. After washing twice with water, the trapped peptides were eluted with 50 μL of 50% ACN containing 0.1% TFA. Finally, 1 μL of the eluate was directly applied for MALDI-TOF MS analysis.
2.8 MALDI-TOF MS analysis
Sample solutions were deposited on the stainless steel plate using the dried droplet method. Briefly, 1 μL of sample solution was applied onto the plate, and then another 1 μL of CHCA matrix solution (5 mg mL−1, 0.1% TFA in 50% ACN solution) was introduced. All MALDI-TOF mass spectra were collected with an Axima TOF2 mass spectrometry (Shimadzu, Kyoto, Japan). The instrument was equipped with a 337 nm nitrogen laser with a 3 ns pulse width. All the mass spectra were performed in positive ion mode with an accelerating voltage of 20 kV. And 200 laser shots were averaged to generate each spectrum. Analysis of peptides and proteins was performed in the reflector and linear TOF detection modes.2.9 RPLC-ESI-MS/MS analysis
The 1D LC-MS/MS analysis was carried out on a hybrid quadrupole-TOF LC-MS/MS mass spectrometer (TripleTOF 5600+, ABSciex) equipped with a nanospray source. Peptides were first loaded onto a C18 trap column (5 mm × 0.3 mm i.d., 5 μm, Agilent Technologies) and then eluted into a C18 analytical column (150 mm × 75 μm i.d., 3 μm, 100 Å, Eksigent). Mobile phase A (3% DMSO, 96.9% H2O, 0.1% formic acid) and mobile phase B (3% DMSO, 96.9% ACN, 0.1% formic acid) were used to establish a 100 min gradient, which was comprised of 0 min in 5% B, 65 min of 5–23% B, 20 min of 23–52% B, 1 min of 52–80% B, 4 min of 80% B, 0.1 min of 80–5% B, and 10 min of 5% B. A constant flow rate was set at 300 nL min−1. MS scans were conducted from 350 to 1500 amu, with a 250 ms time span. For MS/MS analysis, each scan cycle consisted of one full-scan mass spectrum (with m/z ranging from 350 to 1500 and charge states from 2 to 5) followed by 40 MS/MS events. The threshold count was set to 120 to activate MS/MS accumulation and former target ion exclusion was set for 18 s.Data analysis: raw data from TripleTOF 5600+ were analyzed with ProteinPilot Software 4.5. Data were searched against the Uniprot human reference proteome database (version 201408) using the following parameters: sample type, identification; cys alkylation, none; digestion, none. Search effort was set to thorough ID. A 1% Critical False Discovery Rates in ProteinPilot was selected to calculate the number of identifications.
3. Results and discussion
3.1 Synthesis and characterization of MOMS
The synthesis procedure (Fig. 1) was adapted from the modified Stöber method for mesoporous silica spheres. Through a surfactant-template approach, mesostructure CTAB/SiO2 composite was formed within the porous network of pomelo peel. Based on size difference, the pomelo peel/CTAB/SiO2 composite could be easily separated from nanoscale or microscale by-product in the solution by a strainer. After calcination and acid treatment, CTAB and the components of pomelo peel were removed, and then MOMS was obtained.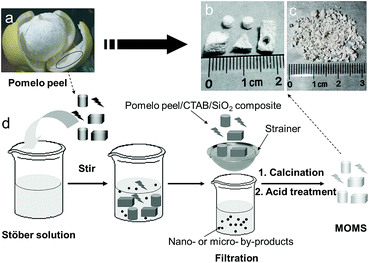 | ||
| Fig. 1 Photographs of pomelo (a), various shapes (b) and small pieces (c) of MOMS. And schematic procedure for preparation of the MOMS (d). | ||
In previous works, the size and shape of monolithic material was usually determined by the size and shape of reaction vessel.14,18,41 Different sizes and shapes of monolithic materials needed to be prepared in different vessels. In this work, the size and shape of MOMS depended on the size and shape of pomelo peel. When different sizes and shapes of pomelo peels were used in one-pot preparation, the corresponding sizes and shapes of MOMS were prepared. As shown in Fig. 1b, special sizes and shapes of MOMS can be prepared simultaneously in one-pot synthesis. In addition, this method can achieve large-scale preparation of small pieces (1–3 mm) of MOMS (Fig. 1c) using pomelo peel fragments, which was minced by a juicer, as the hard template. The sizes of these small pieces of MOMS made them suitable to serve as packed adsorbents for solid phase extraction (SPE). These results demonstrated that the developed method is convenient to prepare mesoporous silica materials with particular shapes for specific applications.
The composition of the materials was examined by energy-dispersive X-ray (EDX) analysis (Fig. S2†). The result showed that pomelo peel was composed of carbon, oxygen and some metallic elements (Fig. S2a†). The main composition of pomelo peel/CTAB/SiO2 was found to be carbon, oxygen and silicon (Fig. S2c†), preliminarily indicating the successful deposition of silica into pomelo peel. Fig. S2d† showed that there existed a few metal salts in the product after calcination of pomelo peel/CTAB/SiO2. To investigate the source of the metal salts, pomelo peel was directly calcined at 550 °C followed by EDX analysis of the ash. Fig. S2b† showed that the ash was composed of metal salts, indicating the metal salts in the crude MOMS product was derived from pomelo peel. It was found that the ash can be quickly dissolved in 6 M HCl. Thus, after acid treatment of the crude MOMS product, pure monolithic silica composed of silicon and oxygen was successfully obtained (Fig. S2e†).
We further examined the morphology of the materials by scanning electron microscopy (SEM). The pomelo peel showed a typical sponge-like appearance (Fig. 2a), which revealed a three-dimensionally (3D) reticulated macroporous architecture (with pore size over 100 μm). The 3D macroporous networks provided ideal habitats for in situ deposition of silica. The image of pomelo peel/CTAB/SiO2 composite demonstrated the successful deposition of silica spheres into the porous network of pomelo peel (Fig. 2b). It can be concluded that the formation of silica sphere in the modified Stöber synthesis seemed to cause their deposition and agglomeration onto the carbohydrate-based surface of pomelo peel. As a result, removal of pomelo peel by calcination resulted in the 3D macroporous silica network. Fig. 2c and d showed that the as-prepared MOMS possessed a continuous skeleton with interconnecting macropores and the primary structure of the MOMS was silica sphere with a size range from 400 to 900 nm. The small size of the primary silica spheres can lead to short-range mesopores, which is propitious to fast equilibrium of molecule transportation.
The mesostructure of MOMS was measured by high-resolution transmission electron microscopy (HR-TEM), nitrogen sorption experiment and small-angle X-ray diffraction (XRD). The HR-TEM image (Fig. S3†) demonstrated that the MOMS had mesoporous channels that arranged perpendicularly to the surface of the spherical unit. The Brumauer–Emmett–Teller (BET) surface area and total pore volume of MOMS were found to be 1120 m2 g−1 and 0.91 cm3 g−1, respectively. N2 sorption–desorption isotherms revealed a type-IV curve for as-prepared MOMS (Fig. 3a), which indicated the presence of mesostructure.42,43 The narrow and sharp pore size distribution curve of MOMS with the center at the mean value of 2.3 nm suggested that the mesopores had very uniform size (Fig. 3a inset). The ordered mesostructure of MOMS was further confirmed by small-angle XRD pattern analysis (Fig. 3b). One high-intensity diffraction peak of (1 0 0) and two additional diffraction peaks of (1 1 0), (2 0 0) were observed, which can be assigned to a two-dimensional hexagonal mesostructure (space group p6mm).44,45 The macroporous property was measured by mercury intrusion porosimetry. As shown in Fig. S4,† MOMS had a broad macropore size distribution ranged from 1 μm to 100 μm, and a large porosity of 83.7%, which demonstrated the successful fabrication of the macropore structure.
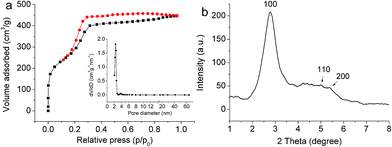 | ||
| Fig. 3 N2 adsorption–desorption isotherms and pore size distribution (the inset) of MOMS (a). The small-angle XRD pattern of MOMS (b). | ||
3.2 Peptide enrichment with MOMS
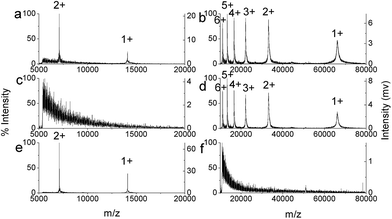
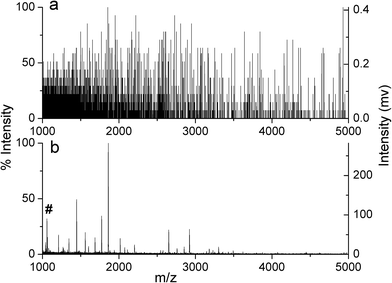
Endogenous peptides play essential roles in many physiological and pathological processes.46–49 Because of the complex matrix of biological sample, selective enrichment of peptides is necessary before mass spectrometry (MS) analysis.50 The main interferences are high abundance of proteins and salts. As reported in previous works, OMS has been applied to enrichment of endogenous peptides based on size-exclusion mechanism and hydrophobic interaction.40,51 On the other hand, to prevent the production of artificial peptides originating from the further degradation of the endogenous proteins by proteases released during treatment, the peptide enrichment should be rapid and easily performed.52 Here, in view of the bulk appearance and special porous structure, small pieces (1–3 mm) of MOMS were packed in a syringe for SPE enrichment of peptides. The detailed procedure of lab-in-syringe SPE (Fig. S1†) was according to our previous work.38 The in-syringe SPE was easy-operation and rapid.BSA digests were used to evaluate the enrichment efficiency of MOMS, as well as OMS microspheres, which was used for the comparison. The result showed that 20 nM BSA digests was hardly detected without any treatment (Fig. 4a). However, after enrichment with MOMS, the signals of the extracted peptides from the eluate were abundant and obvious (Fig. 4c, Table S1†), while no assignable signal was observed in the solution after extraction (Fig. 4b). As shown in Table S1,† 22 or 21 peptides were identified with MOMS or OMS microspheres, respectively. These results demonstrated that the performances of MOMS and OMS microspheres in the peptide enrichment were comparable. However, the analysis process with the in-syringe SPE system was faster and simpler compared to dispersive SPE using OMS microspheres, which required repeated high-speed centrifugation steps.
The size-exclusion effect of the pore structure of MOMS was examined with lysozyme (MW 14.4 kDa), HRP (MW 44.0 kDa) and BSA (MW 66.4 kDa). After enrichment using MOMS, the mass spectrum of lysozyme in the solution after extraction showed no assignable signal (Fig. 5c), whereas, the S/N ratio of protein signal in the eluate (Fig. 5e) significantly increased compared with the direct analysis (Fig. 5a). On the contrary, the mass spectrum of BSA or HRP in the solution after extraction (Fig. 5d and S5b†) was similar to that without any treatment (Fig. 5b and S5a†), while the spectrum of the eluate displayed no assignable signal (Fig. 5f and S5c†). These results demonstrated that MOMS can effectively enrich peptides and low-MW proteins, and exclude high-MW proteins.
MOMS was further applied to enrich peptides from human plasma. Because of the interference of high abundance of salts and proteins,53,54 no assignable peptide peaks were detected by direct analysis of diluted human plasma (Fig. 6a). After enrichment with MOMS, a series of signals in the MW range from 1 to 5 kDa, including the signal of spiked synthetic peptide, can be clearly observed (Fig. 6b). The peptides extracted from plasma with MOMS were further analyzed by 1D liquid chromatography coupled with tandem mass spectrometry (1D LC-MS/MS). As shown in Table S2,† 173 unique peptides were identified. These results show that MOMS has great potential to serve as adsorbent for rapid enrichment of endogenous peptides from complex biological samples.
4. Conclusions
In summary, monolithic ordered mesoporous silica was prepared with modified Stöber method using sponge-like pomelo peel and CTAB as dual templates. The preparation procedure is simple, fast, and cost-effective. With the developed strategy, various and controllable sizes and shapes of MOMS can be large-scale prepared simultaneously in one-pot synthesis. The prepared MOMS possesses continuous skeleton with interconnecting macropores, ordered mesostructure, ultra-high surface area and large pore volume. Moreover, the prepared MOMS was proved to be a useful packing adsorbent for enrichment of endogenous peptides. With the developed method, special sizes and shapes of MOMS can be conveniently fabricated for various potential applications such as electrode, catalysis and nanocasting.Acknowledgements
The authors are grateful for financial support from the National Basic Research Program of China (973 Program) (2013CB910702, 2012CB720601), the National Natural Science Foundation of China (21475098, 91217309), and the Natural Science Foundation of Hubei Province, China (2014CFA002).References
- S. H. Wu, C. Y. Mou and H. P. Lin, Chem. Soc. Rev., 2013, 42, 3862–3875 RSC.
- C. Gerardin, J. Reboul, M. Bonne and B. Lebeau, Chem. Soc. Rev., 2013, 42, 4217–4255 RSC.
- W. Li and D. Zhao, Chem. Commun., 2013, 49, 943–946 RSC.
- Y. Zhang, Z. R. Hong, Y.-P. Ren, D. G. Wang and Y. Yokogawa, J. Non-Cryst. Solids, 2014, 402, 149–152 CrossRef CAS PubMed.
- N. Pal and A. Bhaumik, RSC Adv., 2015, 5, 24363–24391 RSC.
- Y. Wang, A. D. Price and F. Caruso, J. Mater. Chem., 2009, 19, 6451–6464 RSC.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson and E. W. Sheppard, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- B. Lefèvre, A. Galarneau, J. Iapichella, C. Petitto, F. Di Renzo, F. Fajula, Z. Bayram-Hahn, R. Skudas and K. Unger, Chem. Mater., 2005, 17, 601–607 CrossRef.
- S. Chigome, G. Darko and N. Torto, Analyst, 2011, 136, 2879–2889 RSC.
- T. Martin, a. Galarneau, F. Di Renzo, F. Fajula and D. Plee, Angew. Chem., Int. Ed., 2002, 41, 2590–2592 CrossRef CAS.
- A. Galarneau, J. Iapichella, K. Bonhomme, F. DiRenzo, P. Kooyman, O. Terasaki and F. Fajula, Adv. Funct. Mater., 2006, 16, 1657–1667 CrossRef CAS PubMed.
- B. H. Yang, Q. Lu, F. Gao, Q. Shi, Y. Yan, F. Zhang, S. Xie, B. Tu and D. Zhao, Adv. Funct. Mater., 2005, 15, 1377–1384 CrossRef PubMed.
- L. Y. Xu, Z. G. Shi and Y. Q. Feng, Microporous Mesoporous Mater., 2007, 98, 303–308 CrossRef CAS PubMed.
- A. Inayat, B. Reinhardt, H. Uhlig, W. D. Einicke and D. Enke, Chem. Soc. Rev., 2013, 42, 3753–3764 RSC.
- J. H. Smått, S. Schunk and M. Lindén, Chem. Mater., 2003, 15, 2354–2361 CrossRef.
- Y. Zhu, K. Morisato, W. Li, K. Kanamori and K. Nakanishi, ACS Appl. Mater. Interfaces, 2013, 5, 2118–2125 CAS.
- Y. S. Hu, P. Adelhelm, B. M. Smarsly, S. Hore, M. Antonietti and J. Maier, Adv. Funct. Mater., 2007, 17, 1873–1878 CrossRef CAS PubMed.
- C. M. A. Parlett, K. Wilson and A. F. Lee, Chem. Soc. Rev., 2013, 42, 3876–3893 RSC.
- H. Maekawa, J. Esquena, S. Bishop, C. Solans and B. F. Chmelka, Adv. Mater., 2003, 15, 591–596 CrossRef CAS PubMed.
- C. Triantafillidis, M. S. Elsaesser and N. Husing, Chem. Soc. Rev., 2013, 42, 3833–3846 RSC.
- Y. Deng, C. Liu, T. Yu, F. Liu, F. Zhang, Y. Wan, L. Zhang, C. Wang, B. Tu, P. A. Webley, H. Wang and D. Zhao, Chem. Mater., 2007, 19, 3271–3277 CrossRef CAS.
- Z. Sun, Y. Deng, J. Wei, D. Gu, B. Tu and D. Zhao, Chem. Mater., 2011, 23, 2176–2184 CrossRef CAS.
- Z. Y. Yuan and B. L. Su, J. Mater. Chem., 2006, 16, 663–677 RSC.
- S. Tao, Y. Wang and Y. An, J. Mater. Chem., 2011, 21, 11901–11907 RSC.
- J. G. Li, R. B. Lin and S. W. Kuo, RSC Adv., 2013, 3, 17411–17423 RSC.
- Y. Zhou, W. G. Lin, M. M. Wan, J. Yang and J. H. Zhu, J. Mater. Chem., 2012, 22, 23633 RSC.
- Z. G. Shi, Y. Q. Feng, L. Xu, S. L. Da and Y. Y. Ren, Microporous Mesoporous Mater., 2004, 68, 55–59 CrossRef CAS PubMed.
- J. Cai, S. Liu, J. Feng, S. Kimura, M. Wada, S. Kuga and L. Zhang, Angew. Chem., Int. Ed., 2012, 51, 2076–2079 CrossRef CAS PubMed.
- V. Valtchev, M. Smaihi, A. C. Faust and L. Vidal, Angew. Chem., Int. Ed., 2003, 42, 2782–2785 CrossRef CAS PubMed.
- D. van Opdenbosch, G. Fritz-Popovski, O. Paris and C. Zollfrank, J. Mater. Res., 2011, 26, 1193–1202 CrossRef CAS.
- Y. Zhang, X. Liu and J. Huang, ACS Appl. Mater. Interfaces, 2011, 3, 3272–3275 CAS.
- M. Thielen, T. Speck and R. Seidel, J. Mater. Sci., 2013, 48, 3469–3478 CrossRef CAS.
- X. Sun, X. Wang, N. Feng, L. Qiao, X. Li and D. He, J. Anal. Appl. Pyrolysis, 2013, 100, 181–185 CrossRef CAS PubMed.
- P. Feng, X. Bu, G. D. Stucky and D. J. Pine, J. Am. Chem. Soc., 1999, 122, 994–995 CrossRef.
- Y. Zhou, J. H. Schattka and M. Antonietti, Nano Lett., 2004, 4, 477–481 CrossRef CAS.
- B. Pauwels, G. van Tendeloo, C. Thoelen, W. van Rhijn and P. A. Jacobs, Adv. Mater., 2001, 13, 1317–1320 CrossRef CAS.
- Z. Teng, G. Zheng, Y. Dou, W. Li, C. Y. Mou, X. Zhang, A. M. Asiri and D. Zhao, Angew. Chem., Int. Ed., 2012, 51, 2173–2177 CrossRef CAS PubMed.
- G. T. Zhu, X. S. Li, X. M. Fu, J. Y. Wu, B. F. Yuan and Y. Q. Feng, Chem. Commun., 2012, 48, 9980–9982 RSC.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS PubMed.
- R. Tian, H. Zhang, M. Ye, X. Jiang, L. Hu, X. Li, X. Bao and H. Zou, Angew. Chem., Int. Ed., 2007, 46, 962–965 CrossRef CAS PubMed.
- Z. L. Hua, J. H. Gao, W. B. Bu, L. X. Zhang, H. R. Chen and J. L. Shi, J. Sol–Gel Sci. Technol., 2009, 50, 22–27 CrossRef CAS.
- M. M. Wan, J. Y. Yang, Y. Qiu, Y. Zhou, C. X. Guan, Q. Hou, W. G. Lin and J. H. Zhu, ACS Appl. Mater. Interfaces, 2012, 4, 4113–4122 CAS.
- J. Kim, J. E. Lee, J. Lee, J. H. Yu, B. C. Kim, K. An, Y. Hwang, C. H. Shin, J.-G. Park, J. Kim and T. Hyeon, J. Am. Chem. Soc., 2005, 128, 688–689 CrossRef PubMed.
- Y. Deng, D. Qi, C. Deng, X. Zhang and D. Zhao, J. Am. Chem. Soc., 2007, 130, 28–29 CrossRef PubMed.
- L. Zhang, S. Qiao, Y. Jin, H. Yang, S. Budihartono, F. Stahr, Z. Yan, X. Wang, Z. Hao and G. Q. Lu, Adv. Funct. Mater., 2008, 18, 3203–3212 CrossRef CAS PubMed.
- M. Soloviev and P. Finch, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2005, 815, 11–24 CrossRef CAS PubMed.
- Y. Li, X. Zhang and C. Deng, Chem. Soc. Rev., 2013, 42, 8517–8539 RSC.
- M. Zhao, Y. Xie, C. Deng and X. Zhang, J. Chromatogr. A, 2014, 1357, 182–193 CrossRef CAS PubMed.
- M. Zhao, C. H. Deng and X. M. Zhang, ChemPlusChem, 2014, 79, 359–365 CrossRef CAS PubMed.
- Y. Gao, L. Lin, Z. Huang, Y. Chen and W. Hang, Anal. Methods, 2011, 3, 773 RSC.
- G. T. Zhu, X. S. Li, Q. Gao, N. W. Zhao, B. F. Yuan and Y. Q. Feng, J. Chromatogr. A, 2012, 1224, 11–18 CrossRef CAS PubMed.
- V. T. Ivanov, A. A. Karelin and O. N. Yatskin, Pept. Sci., 2005, 80, 332–346 CrossRef CAS PubMed.
- H. Qin, P. Gao, F. Wang, L. Zhao, J. Zhu, A. Wang, T. Zhang, R. a. Wu and H. Zou, Angew. Chem., Int. Ed., 2011, 50, 12218–12221 CrossRef CAS PubMed.
- G. T. Zhu, X. M. He, X. S. Li, S. T. Wang, Y. B. Luo, B. F. Yuan and Y. Q. Feng, J. Chromatogr. A, 2013, 1316, 23–28 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11895f |
| This journal is © The Royal Society of Chemistry 2015 |