Phase-selective synthesis and formation mechanism of CZTS nanocrystals
Bin Zhoua,
Donglin Xia*b and
Youfa Wang*c
aSchool of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, P. R. China
bState Key Laboratory of Silicate Materials for Architectures, Wuhan University of Technology, Wuhan 430070, P. R. China. E-mail: donglinxia@126.com; Fax: +86-27-87669729; Tel: +86-27-87669729
cState Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, P. R. China. E-mail: wangyoufa@whut.edu.cn; Fax: +86-27-87880734; Tel: +86-27-87651852
First published on 12th August 2015
Abstract
In this paper, we present a simple and feasible phase-selective synthetic approach to kesterite-phase and wurtzite-phase CZTS nanocrystals by controlling the concentration of sulfur and DDT. It is found that the sulfur precursor has an effect on the crystal phase formation and phase purity of the as-synthesized CZTS nanocrystals (NCs). With the increase of the amount of DDT substituted sulfur, CZTS NCs gradually evolve from kesterite-structure nanoplates to wurtzite-structure nanorods, and the as-synthesized CZTS NCs become smaller and more uniform. Time-dependent phase evolution results suggest that a small amount of DDT restrains the reactivity of the sulfur precursors, phase composition gradually evolves from Cu1.94S then to (ZnS)x(Cu2SnS3)1−x intermediate compounds and finally to kesterite CZTS NCs. Conversely, a small amount of S accelerates the decomposition of the DDT thiol group, which results in phase composition transformation from Cu1.94S then to (SnS)x(Cu2ZnS3)1−x intermediate compounds and finally to wurtzite CZTS NCs. The present study is a contribution towards understanding the phase-selective formation mechanism of CZTS nanocrystals, and provides a feasible approach to the synthesis of similar materials.
1 Introduction
Cu2ZnSnS4 (CZTS) quaternary compound semiconductors have been attractive candidates for future thin film photovoltaic applications due to their low environmental impact,1,2 low material cost,3,4 suitable band gap (1.0–1.5 eV), and high absorption coefficient (∼104 cm−1).5 According to Shockley–Queisser photon balanced calculations, the theoretical energy conversion efficiency of PV using a light absorber material like CZTS is ∼32%.6 CZTS compounds usually have two types of crystal structures: kesterite and wurtzite, and kesterite is the stable phase.7–9 It is reported that the photoelectric conversion efficiency of sulfo-selenide CZTS (CZTSSe) thin film solar cells with a kesterite structure has reached 12.6%,10 while photoelectric conversion efficiency of CZTS-based thin film solar cells with a wurtzite structure was 4.3%.11 It is well-known that the crystal structure of a light absorber material determines its optoelectronic properties which in turn affect its performance in PV devices. Therefore, it is highly important to synthesize the desired CZTS nanocrystal (NC) structure. However, the formation mechanism and synthetic route of the phase-controlled CZTS nanocrystals have not been fully understood, which is unfavorable to further improve photovoltaic conversion properties.So far, a variety of synthetic routes such as hot injection, solvothermal and hydrothermal approaches were used to synthesize CZTS NCs. Among these methods, hot injection method became the preferred synthesis CZTS nanocrystals because it can feasibly control the nucleation and growth.12–14 It is reported that the reactions of metal salts with elemental sulfur (S) in oleylamine (OLA) at high temperatures usually lead to the formation of thermodynamically stable tetragonal kesterite CZTS nanocrystals.15–19 However, the high reactivity of S–OLA usually results in formation of multiphase and nanocrystals aggregation. Pateter et al.20 reported that the formation mechanism of CZTS kesterite nanoparticles through dodecanethiol route proceeds due to the subsequent formation of different sulphide results. Moreover, Zou et al.15 have reported wurtzite–kesterite phase control synthesis with different reactivity's ODE-S precursors to form CZTS NCs, but it is still not clear whether the Zn-rich nuclei favor the formation of wurtzite CZTS nanocrystals. In contrast to the synthesis of kesterite CZTS nanoparticles using S as the precursor, the NCs synthesized using 1-dodecanethiol (1-DDT) went through a slower phase evolution process for wurtzite CZTS, where the size of the resultant nanoparticles are more uniform.21,22 Liao et al.23 have reported a simple one-pot heating synthesis for preparing wurtzite-CZTS nanocrystals from plasmonic djurleite nuclei strategy. Furthermore, Ajay Singh et al.13 have reported a reproducible solution synthesis using 1-DDT to obtain high-quality monodisperse wurtzite Cu2ZnSnS4 nanorods. Recently, Li et al.24 have synthesized kesterite, wurtzite, and kesterite and wurtzite mixed phase CZTS nanoparticles using elemental S, DDT, and TAA as the sulfur source, respectively. The recent research advances on the growth and formation mechanism of wurtzite- and kesterite-CZTS NCs have reported.25–27 Their experiments show that priority growth and composition of the ternary phase (Cu–Zn–S or Cu–Sn–S) in the synthesis process strongly influence the composition and crystallographic phase of the final CZTS nanocrystals, which can significantly alter the preference for formation different defects and defect complexes, dramatically influence device performance. However, they did not further provide valid evidences on the mechanism of phase transformation between kesterite and wurtzite structures. Therefore more work is still needed to in-depth investigate the growth mechanism of CZTS nanocrystals, especially the effect of the structure of preferentially formed Cu–Zn–S or Cu–Sn–S nanocrystals.
In present work, we report the phase-selective synthesis of CZTS NCs by controlling concentration of sulfur and DDT. The influence of mixed S–DDT concentration on the crystal phase formation and phase purity of CZTS nanocrystals was studied in detail. In order to fully understand the phase-selective growth mechanism, the relation between CZTS NCs growth and reactive time is urgently required to realize phase composition evolution of particular S–DDT combination. It is found that the proportion of two kinds of crystal-phase CZTS structure varied with the proportion of S and DDT, which indicated that relative cation exchange rate between Sn, Zn and Cu is related to the proportion of S and DDT, which plays a determining role in the formation of different CZTS crystallographic phases. The time evolution of CZTS NCs phase composition reveals the possible formation mechanism for kesterite or wurtzite phases.
2 Experimental section
2.1. Chemical reagents
Copper(II) acetylacetonate (Cu(acac)2, Aladdin, 97%), zinc(II) chloride (ZnCl2, Aladdin, 99.95%), tin(II) chloride (SnCl2, Aladdin, 99%), sublimed sulfur (S, Aladdin, 99.95%), 1-dodecanethiol (DDT, Aladdin, 98%), oleylamine (OLA, Aladdin, 80–90%), hexane (C6H14, 97%), and ethanol (CH3CH2OH, 99.7%) were used as received without further purification. Sulfur and DDT were as the sulfur sources, and oleylamine acted as a coordinating ligand and solvent.2.2. Phase-controlled synthesis of kesterite- and wurtzite-CZTS NCs
In a typical reaction procedure,3 2 mmol Cu(acac)2, 1 mmol ZnCl2, 1 mmol SnCl2 and 10 mL oleylamine were dissolved in a 50 mL three-neck round-bottom flask connected to a Schlenk line under magnetic stirring. The reaction mixture was degassed for 30 min to prevent the formation of oxides, then the mixture was slowly raised to 150 °C at heating rate of 10 °C min−1 and maintained for 5 min to form the metal–ligand complex under argon (Ar) gas atmosphere, subsequently preformed S–OLA–DDT precursor was rapidly injected into the Cu–Zn–Sn mixture. After that, the mixture solution was heated to 240 °C at ramp rate of 5 °C min−1 for nucleus formation and held for 15 min for further Ostwald ripening growth. Finally, the obtained solution was cooled down to room temperature, ethanol was added to each of the aliquots to isolate nanocrystals precipitate, and CZTS nanocrystals are collected by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 8 min. The supernatant was decanted, while the precipitates were dispersed in the hexane and further purified by ethanol several times. Final products were obtained and dried in a vacuum oven overnight at room temperature for further characterization. 1 mol L−1 of OLA–S solution was prepared by dissolving/mixing 10 mmol S with 10 mL of OLA at room temperature, and the S–OLA–DDT mixture solution was obtained by mixing DDT with S–OLA solution at a certain proportion. VDDT/(VDDT + VS) of the S–OLA–DDT precursor was set to 0, 0.25, 0.5, 0.75, and 1 with the total constant concentration of VDDT + VS = 4, where VDDT and VS represent the volume of DDT and volumetric concentration of S dissolved in oleylamine, respectively. Moreover, aliquots of the reaction mixtures were taken with syringe at different times to observe the growth kinetics of CZTS NCs during the reaction for VDDT/(VDDT + VS) = 0.25 and 0.75, respectively.
000 rpm for 8 min. The supernatant was decanted, while the precipitates were dispersed in the hexane and further purified by ethanol several times. Final products were obtained and dried in a vacuum oven overnight at room temperature for further characterization. 1 mol L−1 of OLA–S solution was prepared by dissolving/mixing 10 mmol S with 10 mL of OLA at room temperature, and the S–OLA–DDT mixture solution was obtained by mixing DDT with S–OLA solution at a certain proportion. VDDT/(VDDT + VS) of the S–OLA–DDT precursor was set to 0, 0.25, 0.5, 0.75, and 1 with the total constant concentration of VDDT + VS = 4, where VDDT and VS represent the volume of DDT and volumetric concentration of S dissolved in oleylamine, respectively. Moreover, aliquots of the reaction mixtures were taken with syringe at different times to observe the growth kinetics of CZTS NCs during the reaction for VDDT/(VDDT + VS) = 0.25 and 0.75, respectively.
2.3. Characterization
The crystal structure, composition, and optical properties of the as-synthesized CZTS nanocrystals were characterized by using powder X-ray diffraction (XRD), transmission electron microscopy (TEM)/high-resolution TEM (HRTEM), Raman spectroscopy, energy dispersive X-ray spectroscopy (EDS) and UV-Vis absorption spectroscopy. X-ray powder diffraction (XRD) patterns were collected at a scanning rate of 10° min−1 with a measurement time of 2.0 s per step using a Bruker Discover D8 high-resolution diffractometer employing Cu Kα radiation (λ = 1.5418 Å) over the range of 2θ = 20–70°. Transmission electron microscopy (TEM) and high resolution TEM (HRTEM) images were measured using a JEM 2100F high resolution electron microscope (0.23 nm point-to-point) at an accelerating voltage of 200 kV, equipped with a Gatan slow-scan CCD camera (model 794). The powders were dispersed in an ethanol solution and deposited on a holey carbon film. Raman scattering spectroscopy was measured using Renishaw inVia Raman microscope. All Raman scattering measurements were performed using an excitation wavelength of 514.5 nm argon ion laser with power intensity of 20 mW cm−2. The samples were measured at a scanning rate of 20° min−1 in the over the wavelength range from 200 to 600 cm−1. Energy dispersive X-ray spectroscopy (EDS) was carried out with a Hitachi S-4800 field emission scanning electron microscope. EDS data were collected to analyze elemental changes using a scanning electron microscope (SEM) with an accelerating voltage of 20 keV.3 Results and discussion
3.1. Phase-selective synthesis of CZTS nanocrystals
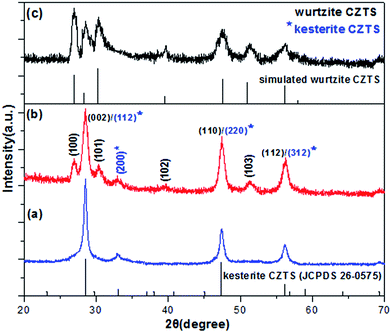
S and DDT are often chosen as the sulfur source in the synthesis of tetragonal kesterite or metastable hexagonal wurtzite CZTS nanocrystals.15,27 Accordingly, we use a simple and feasible hot injection method to synthesize the phase-controlled CZTS nanocrystals by adjusting the proportion of S and DDT as mixing sulfur source.The three typical XRD patterns of as-synthesized CZTS NCs samples with different ratios of VDDT/(VDDT + VS) are shown in Fig. 1. For the sake of comparing and analyzing, the standard XRD patterns of kesterite CZTS (JCPDS no. 26-0575) and the simulated XRD pattern of wurtzite CZTS are also shown in Fig. 1.
Fig. 1a shows XRD pattern of pure tetragonal phase CZTS NCs with VDDT/(VDDT + VS) = 0. The as-synthesized CZTS NCs can be matched very well with the standard pattern of kesterite CZTS (JCPDS no. 26-0575). The XRD patterns appear four major peaks at 28.49°, 32.98°, 47.42°, and 56.20°, corresponding to (112), (200), (220), and (312) lattice planes, respectively. The results are in good agreement with the previously reported data for kesterite CZTS.8 Fig. 1c shows different XRD pattern of hexagonal phase CZTS NCs with VDDT/(VDDT + VS) = 1. These obvious XRD peaks can be observed at 26.93°, 28.49°, 30.35°, 39.66°, 47.42°, 51.20°, and 56.25°. It is clearly noticed that the three diffraction peaks about 28.49°, 47.42°, and 56.25° are almost at the same peaks of kesterite or wurtzite CZTS structure, which indicates that the sample can be attributed to (112), (220), and (312) planes of kesterite CZTS or (002), (110), and (112) planes of wurtzite CZTS, respectively. The diffraction peaks that appear at 26.93°, 30.35°, 39.66°, and 51.20° are attributed to (100), (101), (102), and (103) planes, which indicate that this sample is essentially metastable wurtzite CZTS. Interestingly, Fig. 1b shows XRD pattern of CZTS NCs with VDDT/(VDDT + VS) = 0.5, which is almost similar to the XRD pattern of hexagonal wurtzite phase CZTS NCs with VDDT/(VDDT + VS) = 1. At the same time, the three strong diffraction peaks and one weak peak corresponding to 28.52°, 47.42°, 56.26°, and 32.92° can be matched very well with the standard pattern of kesterite CZTS (JCPDS no. 26-0575). From above experimentally observed results analysis, it can be concluded that CZTS nanocrystals contain the crystal structure of both kesterite phase and wurtzite phase. With the increase of the ratio of VDDT/(VDDT + VS), the strongest diffraction peak of the kesterite CZTS (112) plane was gradually decreased, (200) plane also became weaker and weaker until it completely disappeared. In addition, the diffraction peak of the wurtzite CZTS (100), (101), (102), and (103) plane became stronger and stronger. These results indicated that phase-controlled of CZTS NCs gradually evolves from pure kesterite to pure wurtzite with the increase of the ratio of VDDT/(VDDT + VS).
Fig. 2 shows TEM and HRTEM images of the corresponding CZTS NCs synthesized with different ratios of VDDT/(VDDT + VS). As can be seen from Fig. 2a and b, the as-synthesized CZTS NCs have irregular shape with good polycrystallinity and possess an average size of 20 ± 5 nm. The interplanar spacing (d-spacing) measured from an average of at least 10 planes in the HRTEM image was determined to be 3.1 Å for the (112) planes which angle was measured to be 62°, and well defined anion stacking sequence of ABC/ABC21 (viewed along the [112] direction), as shown in Fig. 2b.
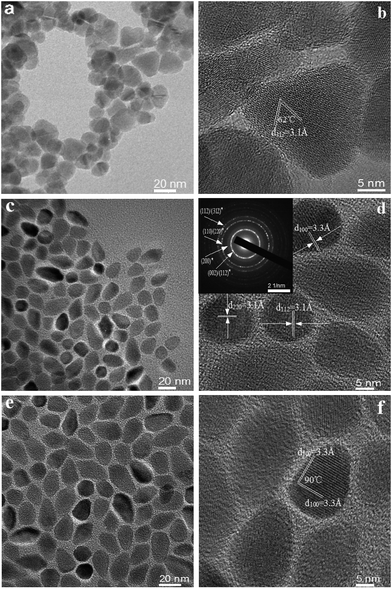 | ||
| Fig. 2 TEM images of as-synthesized CZTS NCs with VDDT/(VDDT + VS) ratios of (a) 0, (c) 0.5, (e) 1; HRTEM images of the corresponding CZTS NCs with VDDT/(VDDT + VS) ratios of (b) 0, (d) 0.5, (f) 1. The inset shows selected area electron diffraction (SAED) image in Fig. 2d. | ||
The TEM image of as-synthesized CZTS NCs with VDDT/(VDDT + VS) = 0.5 possesses two kinds of shape of nanocrystals. The grain size of ellipsoid-shaped nanorods is 24 nm ± 2.5 nm in length and 13 ± 4 nm in diameter, while the grain size of circular nanoplates is measured to be 14 ± 2 nm in diameter, as shown in Fig. 2c. The HRTEM images in Fig. 2d again confirm the coexisting phase CZTS NCs containing kesterite circular nanoplates and wurtzite ellipsoid nanorods. The lattice spacings were found to be 1.9 and 3.2 Å, which correspond to the lattice spacing of (220) planes and (112) planes, respectively, for kesterite CZTS NCs. And the lattice spacing was determined to be 3.3 Å for the (002) planes, for wurtzite CZTS NCs. To prove the presence of kesterite and wurtzite two-type phase structure, the crystal structure of CZTS was also confirmed by analysis of Selected-Area Electron Diffraction (SAED) pattern as shown in Fig. 2d. CZTS NCs synthesized with VDDT/(VDDT + VS) = 1 have single crystal structure, and present uniform nanorod-shape with an average size of 28 nm ± 2.5 nm in length and 18 ± 4 nm in diameter, as shown in Fig. 2e. A lattice spacing of the HRTEM image in the Fig. 2f was measured 3.3 Å in the shape of nanorod and corresponds to the (002) plane of the wurtzite structure. The angle between the (002) and (002) planes was measured to be 90°, which reveals the atom stacking arrangement (AB/AB stacking) in a hexagonal crystal system.21
Based on our experiments, S and DDT resource precursors play a decisive role in phase selective synthesis from the tetragonal nanoplates to hexagonal nanorods. As can be seen from HRTEM images, kesterite structure nanocrystals exist in the shape of triangular and round shape nanoplates, while hexagonal wurtzite phase structure nanocrystals present in the shape of ellipsoid nanorods, kesterite- and wurtzite-phase two types structure were co-existence in the nanoplates and nanorods grain. These results further reveal that the morphology and crystalline phase structure of CZTS NCs can be controlled by adjusting the ratio of VDDT/(VDDT + VS). DDT as both a sulfur source and a ligand in the reaction minimizes the surface area of the high energy (112) plane and promotes that (100) nanocrystal facet preferentially grow, which may explain the phase transition process from kesterite phase to wurtzite phase, conformed by XRD patterns of Fig. 1.
The element compositions of the as-synthesized CZTS NCs samples analyzed by EDS are shown in Table 1.
| VDDT/(VDDT + VS) | Cu (at%) | Zn (at%) | Sn (at%) | S (at%) | Sn/Zn | Cu/(Zn + Sn) |
|---|---|---|---|---|---|---|
| 0 | 26.89 | 12.67 | 15.76 | 47.34 | 1.24 | 0.94 |
| 0.25 | 26.47 | 12.28 | 14.73 | 48.26 | 1.20 | 0.98 |
| 0.5 | 26.65 | 13.36 | 14.56 | 48.87 | 1.09 | 0.95 |
| 0.75 | 26.43 | 15.21 | 14.29 | 49.18 | 0.94 | 0.89 |
| 1 | 26.62 | 15.49 | 13.41 | 49.76 | 0.86 | 0.92 |
As can be seen from Table 1, the element composition of the as-synthesized CZTS NCs samples changed little with the increase of the ratio of VDDT/(VDDT + VS). The coexisting kesterite and wurtzite CZTS NCs sample with VDDT/(VDDT + VS) = 0.5 shows an average Cu/Zn/Sn/S ratio of approximately 1.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.09
1.09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.67, which is close to the stoichiometric ratio reported by Khare et al.25 The as-synthesized CZTS samples were Cu-poor as the composition ratio of Cu/(Zn + Sn) was no more than 1 even though the proportion of VDDT/(VDDT + VS) increased, as shown in Fig. 3. Interestingly, it is can be clearly seen that there are significant composition differences between kesterite and wurtzite structure CZTS NCs samples. The obtained CZTS NCs samples are Sn-rich but Zn-poor when the ratio of VDDT/(VDDT + VS) changes from 0 to 0.5. The results suggest that Sn preferentially interacts with Cu and produces Cu–Sn–S transitional phase. Conversely, CZTS NCs samples for VDDT/(VDDT + VS) = 0.75–1 is very Zn-rich, suggesting that Cu possibly preferentially interacts with Zn and forms Cu–Zn–S transitional phase, as shown in Sn/Zn sparklines of Fig. 3.
3.67, which is close to the stoichiometric ratio reported by Khare et al.25 The as-synthesized CZTS samples were Cu-poor as the composition ratio of Cu/(Zn + Sn) was no more than 1 even though the proportion of VDDT/(VDDT + VS) increased, as shown in Fig. 3. Interestingly, it is can be clearly seen that there are significant composition differences between kesterite and wurtzite structure CZTS NCs samples. The obtained CZTS NCs samples are Sn-rich but Zn-poor when the ratio of VDDT/(VDDT + VS) changes from 0 to 0.5. The results suggest that Sn preferentially interacts with Cu and produces Cu–Sn–S transitional phase. Conversely, CZTS NCs samples for VDDT/(VDDT + VS) = 0.75–1 is very Zn-rich, suggesting that Cu possibly preferentially interacts with Zn and forms Cu–Zn–S transitional phase, as shown in Sn/Zn sparklines of Fig. 3.
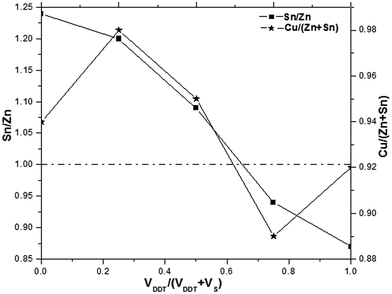 | ||
| Fig. 3 Evolutions of the Sn/Zn and Cu/(Zn + Sn) of CZTS NCs with different ratio of VDDT/(VDDT + VS). | ||
It is reported that all proposed formation mechanism of wurtzite- and kesterite-CZTS NCs involves the nucleation of CuxS,23 and the interdiffusion of Zn and Sn.12 The EDS results suggest that the change of VDDT/(VDDT + VS) has a substantial impact on ternary phase (Cu–Sn–S or Cu–Zn–S) nuclei, which possibly further affect the phase transition of CZTS. Therefore, the effect of Cu–Zn–S or Cu–Sn–S transitional phase on the growth of CZTS nanocrystals needs to be studied.
3.2. Phase-selective formation mechanism of CZTS nanocrystals
It is reported that Cu–Sn–S intermediate compounds in the CZTS phase evolution process is contributed to formation of kesterite NCs synthesized by elemental sulfur.17 However, there is lack of sulfur role in phase-selective growth. Few reports explain that Cu–Zn–S intermediate compounds exist in the wurtzite phase evolution process. According to our experimental results, CZTS phase transitions are closely related to the ratio of VDDT/(VDDT + VS).Fig. 4 shows the color change of the reaction solution with different ratio of VDDT/(VDDT + VS) after injection 5 min. As observed in the Fig. 4a, the mixture solution for VDDT/(VDDT + VS) = 0 immediately becomes dark brown because H2S releases rapidly. In comparison, the solution color for VDDT/(VDDT + VS) = 0.25 slowly changes to dark yellow, indicating that small amounts of DDT slightly restrain the reaction reactivity of sulfur, as observed in the Fig. 4b. Moreover, the mixture solution for VDDT/(VDDT + VS) = 0.75 presented bright orange, indicating that a little S helps to improve reaction activity by accelerating the decomposition of the DDT thiol group, as shown in the Fig. 4c. During heating the solution turned from a dark to a bright orange color, suggesting the formation nanocrystals possessed different structure with various ratio of VDDT/(VDDT + VS).
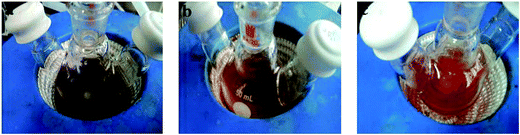 | ||
| Fig. 4 Three color photograph corresponding to VDDT/(VDDT + VS) = (a) 0, (b) 0.25, and (c) 0.75, respectively, after injection different ratio S and DDT for 5 min. | ||
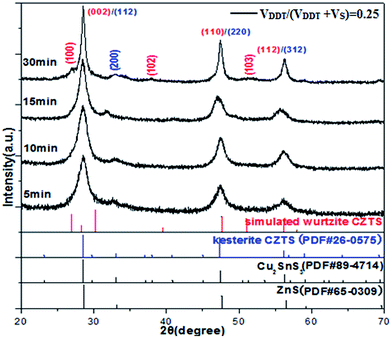
In order to fully understand the phase-selective growth mechanism for CZTS nanocrystals synthesized with different ratio of VDDT/(VDDT + VS), more experiments focus on the phase composition evolution under the condition of VDDT/(VDDT + VS) = 0.25 and 0.75, respectively.
As can be seen from Fig. 5, the diffraction peaks of XRD patterns for the 30 min reaction time can be observed at 28.54°, 32.93°, 47.45°, and 56.25°, corresponding to (112), (200), (220), and (312) lattice planes, respectively. The diffraction peaks match well with the standard XRD pattern for kesterite CZTS (PDF# 26-0575). In addition, weak diffraction peaks also appear at 26.94°, 37.95°, and 51.54°, corresponding to (100), (102), and (103) planes, which were in good agreement with that of the simulated wurtzite CZTS. However, the crystal structures of the nanoparticles identified by XRD were tetragonal kesterite structure and each diffraction peak was located at almost the same diffraction angle even as early as 5 min, 10 min, and 15 min after the injection.
Since XRD patterns of sulphides such as ZnS and Cu2SnS3 (CTS) are similar with CZTS because of their similarity in the crystal structure. Therefore, it is not sure that their diffraction peaks could be attributed to binary or ternary impurity phases due to the overlapping diffraction peaks of the standard XRD patterns of ZnS (PDF# 65-0309) and CTS (PDF# 89-4714).
In order to understand CZTS phase evolution process and distinguish kesterite CZTS from tetragonal CTS and cubic ZnS, Raman spectroscopy was further used to detect compositional phase of samples for different reactive time. Raman spectra of as-synthesized CZTS NCs with VDDT/(VDDT + VS) = 0.25 at (a) 5 min, (b) 10 min were shown in Fig. 6. As can be seen from Fig. 6a, a broad peak at 264 cm−1 corresponded to the Cu1.94S phase, while a weak peak at 349 cm−1 corresponded to the cubic ZnS phase. When the reactive time increased to 10 min, three Raman peaks presented at 221, 302, and 359 cm−1, respectively. These peaks corresponded to the SnS phase and the cubic CTS phase, as can be observed from Fig. 6b. The results reveal that compositional phase gradually evolutes from Cu1.94S then to (ZnS)x(Cu2SnS3)1−x intermediate compounds finally to CZTS NCs.
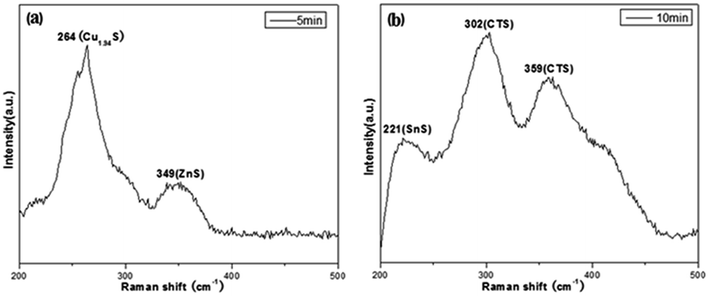 | ||
| Fig. 6 Raman scattering spectra of as-synthesized typical CZTS NCs with VDDT/(VDDT + VS) = 0.25 for (a) 5 min, (b) 10 min. | ||
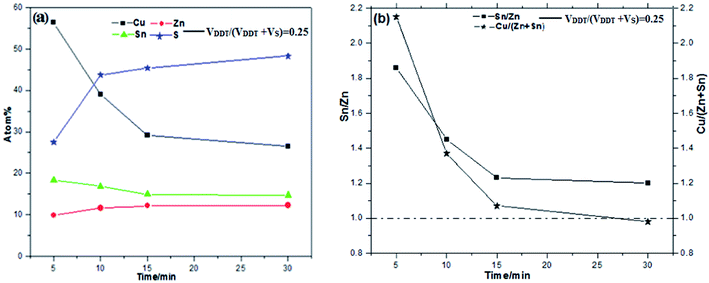
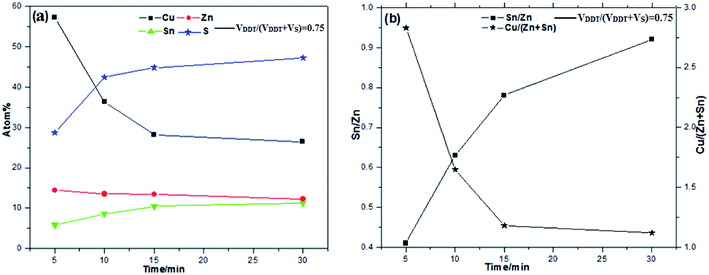
The elemental atomic percent and ratios of Sn/Zn and Cu/(Zn + Sn) in the CZTS NCs samples with different reaction time were shown in Fig. 7a and b, respectively.
As can be seen from Fig. 7a, the element composition atomic percent varied with reaction time. The amount of S increases whiles the amount of Cu decreases as the reaction progress. When the reaction time is less than 15 min the amount of Sn decreases while the amount of Zn increases. The amount of Sn and Zn almost has on change when the reaction time is more than 15 min. The results suggest the earliest formation particles may be a Cu–Sn–S compound, as confirmed by Cu/(Zn + Sn) sparkline of Fig. 7b. In addition, from Fig. 7b we also see that the Sn/Zn ratio slowly changed from 1.86 to 1.20 with the time increases, which is greater than the theoretical value of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ratio of Sn/Zn decreases may confirm that introduction of DDT reduces sulfur reactivity. The experiment results suggest that Sn preferentially reacted with Cu during the reaction process, and possible Cu–Sn–S compound or alloy were produced. Finally, most kesterite monodispersed CZTS NCs with small wurtzite particle were formed.
1. The ratio of Sn/Zn decreases may confirm that introduction of DDT reduces sulfur reactivity. The experiment results suggest that Sn preferentially reacted with Cu during the reaction process, and possible Cu–Sn–S compound or alloy were produced. Finally, most kesterite monodispersed CZTS NCs with small wurtzite particle were formed.
Fig. 8 show the TEM image of monodispersed CZTS nanocrystals for VDDT/(VDDT + VS) = 0.25 with different reactive time. (a) 5 min, (b) 30 min.
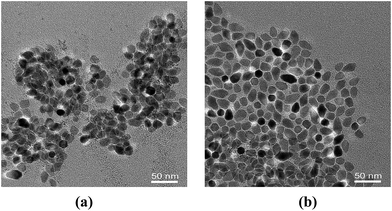 | ||
| Fig. 8 TEM images of as-synthesized typical CZTS NCs for VDDT/(VDDT + VS) = 0.25 with different reactive time. (a) 5 min, (b) 30 min. | ||
In an earlier work, kesterite CZTS NCs were synthesized by using S as the resource, and it was found that sulfur and the amine group could react to form alkyl ammonium polysulfides at room temperature.19 However, the wurtzite CZTS nanoparticle synthesized using DDT went through a slower phase evolution process via a binary phase due to DDT molecule contains a 12 carbon chain and a thiol headgroup.28 Therefore, we believe that the presence of small amounts of DDT may restrain the reaction of highly reactive sulfur, and further slow the nucleation rate of CZTS NCs, which was proved by the observation of ZnS and CTS. There seems to support phase selective synthesis of feasibility by using sulfur and 1-DDT resource.
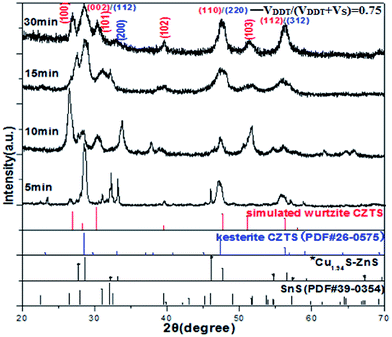
XRD patterns for sample VDDT/(VDDT + VS) = 0.75 taken out from reaction mixtures at the time intervals of 5, 10, 15, and 30 min are shown in Fig. 9.
By XRD analysis software Jade 6, two different XRD patterns exist in the CZTS sample 5 min of reaction time, which indicated the formation of two compositional phases. Some XRD diffraction peaks can be observed at 28.54°, 32.93°, 47.45°, and 56.25°, whose peaks position located between the standard XRD patterns of cubic Cu1.94S (PDF# 89-2072) and cubic ZnS (PDF# 65-0309), which suggested that the particles may be Cu–Zn–S compounds, which is in good agreement with the previously reported Cu1.94S–ZnS compounds.30,31 Apart from these XRD diffraction peaks, others exhibit a good match for standard XRD pattern peaks of orthorhombic SnS (PDF# 39-0354). Compared with weak diffraction peaks of SnS, intense diffraction peaks suggest that Cu1.94S and ZnS may preferentially form over SnS. Moreover, two different crystalline phase nuclei (cubic and orthorhombic phase) have a potential impact on hexagonal wurtzite CZTS NCs. These results seem to give a reasonable explanation that the Zn-rich nuclei favor the formation of hexagonal wurtzite CZTS nanocrystals, which has not been identified until now. With the reaction time prolonged, XRD pattern peaks of orthorhombic SnS gradually disappear, some pattern peaks located at (002) and (200) crystal planes slowly weakened. In contrast, some diffraction peaks were gradually observed at 26.94°, 30.35°, 39.58°, 47.42°, and 56.26° corresponding to (100), (101), (102), (220), and (312) lattice planes, respectively, as shown in the Fig. 9.
For sample 30 min of reaction time, the experimental XRD pattern is consistent with the simulated wurtzite CZTS. In addition, the small unique peak (200) plane is specifically matched with the standard XRD pattern for kesterite CZTS (PDF# 26-0575), indicating that final sample contains slight kesterite CZTS NCs. These results further reveal compositional phase evolution mechanism of kesterite CZTS. A possible formation mechanism of kesterite CZTS was described as follows: because of much higher affinity of Cu2+ over Zn2+ and Sn4+, Cu2+ in Cu-based Cu1.94S binary nuclei first form with S–H or S,22 which have a relatively high mobility, which can facilitate an exchange with other metal ions. Owing to the ionic radius of Zn2+ (74 pm) being closer to that of Cu+ (77 pm) than Sn4+ (69 pm), Zn2+ will be preferentially doped into Cu1.94S over Sn4+.29 Moreover, DDT as strong coordinating ligand can strongly bind the metal precursors at low temperatures, prevent from the interaction of Cu and Sn.27 With the increase of temperature, the unstable ZnS binary nuclei result in the diffusion of Zn2+ into Cu1.94S nanoparticles to form Cu–Zn–S compound. With the reaction progress, the concentration of Cu+ and Zn2+ both decrease as Sn is incorporated, zinc content gradually reaches the theoretical value, which induce the relatively high doping speed of tin, and then subsequently the diffusion of Sn4+ into Cu–Zn–S nanoparticles led to the formation of kesterite nanocrystals, evidence by the disappearance of orthorhombic SnS phase, suggesting that formation of phase composition between orthorhombic SnS and cubic Cu–Zn–S compound (or Cu1.94S and ZnS) is necessary for the formation of wurtzite CZTS nanocrystals in our reaction system.
Fig. 10a and b show the element composition and the cation ratios of Cu/(Zn + Sn) and Zn/Sn in the CZTS NCs with VDDT/(VDDT + VS) = 0.75 as a function of reaction time, respectively. Fig. 10a clearly shows that the amount of Zn is decreasing compared to the amount of Sn in the nanoparticles over time. In the whole reaction process, the amount of S increases and the amount of Cu decreases, respectively. The results suggested the earliest particles may be a Cu–Zn–S compound (or Cu1.94S and ZnS), which was further confirmed by Cu/(Zn + Sn) evolutions. In addition, the steep rise of Sn/Zn fractions may again confirm that introduction of S accelerates the reaction of DDT, so that the phase nucleation rate is slightly faster than the reaction of pure DDT. The observation results suggest that Zn preferentially acts with Cu, possibly forms Cu–Zn–S compound or alloy particles. Finally, most wurtzite CZTS NCs were formed with small kesterite particle. As the reaction rate of DDT slowed down, the growth process is controlled by the bond-breaking process to release S, and therefore a nearly monodispersed size distribution was obtained, as shown in the Fig. 11. Our experiment results agree well with a previous report,7 phase selectivity for CZTS depends on the reactivity of Zn and S precursors.
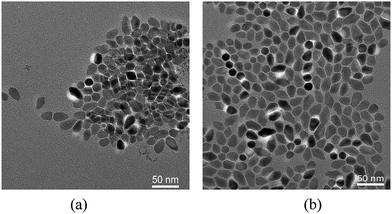 | ||
| Fig. 11 TEM images of as-synthesized CZTS NCs for VDDT/(VDDT + VS) = 0.75 with different reactive time. (a) 5 min, (b) 30 min. | ||
Based on the above experimental analysis, the growth mechanism and phase-selective synthesis of CZTS nanocrystals using elemental S and DDT as mixing sulfur source were proposed in this paper, as shown in Fig. 12.
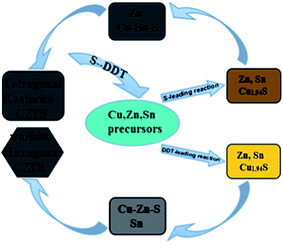 | ||
| Fig. 12 The schematic diagram of formation mechanism and phase-selective synthesis route of CZTS nanocrystals. | ||
For compositional evolution of CZTS NCs sample with VDDT/(VDDT + VS) = 0.25, mixture solution color slowly changed from opaque light blue to dark brown due to the dissolution of copper acetylacetonate. The sulfur dominant mixture solution slowly changed into dark yellow compared with pure sulfur solution, which indicated that small amounts of DDT restrain the reaction of highly reactivity sulfur, which provides a strong condition for compositional evolution of kesterite CZTS NCs. Further, CTS is contributed to the formation of kesterite CZTS NCs which indicates Cu–Sn preferred exchange process. In addition, it is also possible that the formation of Cu–Zn–S compounds as early as Cu–Zn exchange contributed to the formation of wurtzite CZTS NCs though nucleation rate of Cu–Sn exchange is much faster than Cu–Zn.
Apparently, cation relative exchange rate between Sn/Zn and Cu1.94S phase was the key to formation of kesterite or wurtzite CZTS NCs. The DDT-leading mixture solution showed bright orange compared with S dominant mixture solution, indicating that little S helps to improve reaction activity by accelerating the decomposition of the DDT thiol group, which were observed from the experiment of VDDT/(VDDT + VS) = 0.75. Namely, abundant DDT strongly hindered preferred exchange process between Cu and Sn, and promoted Cu–Zn priority exchanges, and resulted in Cu–Zn–S compound formation, eventually led to formation with large amounts of wurtzite CZTS NCs. In a word, these efforts profoundly not only reveal the reaction mechanism of S and DDT but also easily realize phase synthesis by changing the proportion of S and DDT.
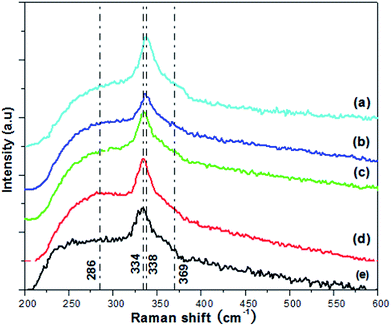
The as-synthesized CZTS samples were measured to prove formation mechanism of phase-selective synthesis. The Raman spectra of the different proportion of S and DDT are shown in Fig. 13. The Raman spectra for VDDT/(VDDT + VS) = 0 exhibits a strong peak and two weak peaks at 338 cm−1, 286 cm−1, and 369 cm−1, respectively, which match well to that of reported by Fernandes et al.32 Raman peak for kesterite CZTS located at 338 cm−1, while weak peak positions located at 288 and 368 cm−1. With the increase of proportion of VDDT/(VDDT + VS), the strongest Raman peak slightly shifts from 338 to 334 cm−1, which indicated formation of the kesterite or wurtzite CZTS NCs. Raman peaks at 334 cm−1 of the sample for VDDT/(VDDT + VS) = 1 also agreed well with the result of Singh et al.,13 who reported a strong Raman peak at 333 cm−1 for wurtzite CZTS nanorods. The Raman results confirm the formation of quaternary CZTS NCs rather than binary or ternary impurities. This is because no noticeable peaks corresponding to Cu1.94S (264 cm−1), ZnS (273 and 348 cm−1), or cubic Cu2SnS3 (267, 303 and 356 cm−1) can be observed.8
4 Conclusions
In summary, we have successfully synthesized the phase-controlled CZTS NCs by adjusting the ratio of S and DDT as mixing sulfur source. It is found that the sulfur precursor ratio of VDDT/(VDDT + VS) in the hot-injection reaction determines the formation and phase purity of the synthesized CZTS NCs. With the increase of the amount of DDT substitute sulfur, CZTS NCs gradually evolve from kesterite-structure nanoplates to wurtzite-structure nanorods, and the as-synthesized CZTS NCs become smaller and more uniform. Time-dependent phase evolutions results suggest that a small amount of DDT restrains sulfur reactivity, phase composition gradually evolutes from Cu1.94S then to (ZnS)x(Cu2SnS3)1−x intermediate compounds finally to kesterite CZTS NCs. In contrast, a small amount of S accelerates the decomposition of the DDT thiol group, which result in compositional phase transformation from Cu1.94S then to (SnS)x(Cu2ZnS3)1−x intermediate compounds finally to wurtzite CZTS NCs. This present study is contributed to understand phase-selective formation mechanism of CZTS nanocrystals, and provides a feasible approach for synthesis of similar materials.Acknowledgements
This work was supported by Wuhan Municipal Science and Technology Bureau, China (grant No. 2015010101010006), National College Students' innovation and entrepreneurship training plan of China (20141049701020), self-determined and innovative research funds from Wuhan University of Technology(grant number 146801006).Notes and references
- C. R. Shannon, A. P. Bruce and L. P. Amy, J. Am. Chem. Soc., 2009, 131, 12054–12055 CrossRef PubMed.
- T. K. Todorov, K. B. Reuter and D. B. Mitzi, Adv. Mater., 2010, 22, E156–E159 CrossRef CAS PubMed.
- Q. Guo, G. M. Ford, W. Yang, B. C. Walker, E. A. Stach, H. W. Hillhouse and R. Agrawal, J. Am. Chem. Soc., 2010, 132, 17384–17386 CrossRef CAS PubMed.
- T. K. Todorov, S. J. Tang, B. O. Gunawan, T. Gokmen, Y. Zhu and D. B. Mitzi, Adv. Energy Mater., 2013, 3, 34–38 CrossRef CAS PubMed.
- J. W. Cho, A. Ismail, S. J. Park, W. Kim, S. Yoon and B. K. Min, ACS Appl. Mater. Interfaces, 2013, 5, 4162–4165 CAS.
- Q. Guo, H. W. Hillhouse and R. Agrawal, J. Am. Chem. Soc., 2009, 131, 11672–11673 CrossRef CAS PubMed.
- M. R. T. Joel, H. L. Yih, P. Srikanth, B. Tom, Y. L. Xing and H. W. Lydia, J. Am. Chem. Soc., 2014, 136, 6684–6692 CrossRef PubMed.
- N. Hiroyasu, K. Susumu and T. Tsukasa, J. Phys. Chem. C, 2013, 117, 21055–21063 Search PubMed.
- V. T. Tiong, Y. Zhang, J. Bella and H. X. Wang, CrystEngComm, 2014, 16, 4306–4313 RSC.
- W. Wei, T. W. Mark, G. Oki, G. Tayfun, K. T. Teodor, Z. Yu and B. M. David, Adv. Energy Mater., 2014, 4, 1301465 Search PubMed.
- W.-C. Yang, C. K. Miskin, C. J. Hages, E. C. Hanley, C. Handwerker, E. A. Stach and R. Agrawal, Chem. Mater., 2014, 26, 3530–3534 CrossRef CAS.
- Y.-X. Wang, M. Wei, F.-J. Fan, T.-T. Zhuang, L. Wu, S.-H. Yu and C.-F. Zhu, Chem. Mater., 2014, 26, 5492–5498 CrossRef CAS.
- A. Singh, H. Geaney, F. Laffir and K. M. Ryan, J. Am. Chem. Soc., 2012, 134, 2910–2913 CrossRef CAS PubMed.
- W.-C. Yang, C. K. Miskin, C. J. Hages, E. C. Hanley, C. Handwerker, E. A. Stach and R. Agrawal, Chem. Mater., 2014, 26, 3530–3534 CrossRef CAS.
- Y. Zou, X. Su and J. Jiang, J. Am. Chem. Soc., 2013, 135, 18377–18384 CrossRef CAS PubMed.
- C. A. Cattley, C. Cheng, S. M. Fairclough, L. M. Droessler, N. P. Young, J. H. Warner, J. M. Smith, H. E. Assender and A. A. R. Watt, Chem. Commun., 2013, 49, 3745–3747 RSC.
- M. D. Regulacio, C. Ye, S. H. Lim, M. Bosman, E. Ye, S. Chen, Q.-H. Xu and M.-Y. Han, Chem.–Eur. J., 2012, 18, 3127–3131 CrossRef CAS PubMed.
- A. Salant, M. Shalom, Z. Tachan, S. Buhbut, A. Zaban and U. Banin, Nano Lett., 2012, 12, 2095–2100 CrossRef CAS PubMed.
- K.-C. Wang, P. Chen and C.-M. Tseng, CrystEngComm, 2013, 15, 9863–9868 RSC.
- A. Pateter, W. Haas, B. Chernev, B. Kunert, R. Resel, F. Hofer, G. Trimmel and T. Rath, Mater. Chem. Phys., 2015, 149–150, 94–98 CrossRef CAS PubMed.
- S. Chen, A. Walsh, Y. Luo, J.-H. Yang, X. Gong and S.-H. Wei, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 159904 CrossRef.
- R. Mainz, A. Singh, S. Levcenko, M. Klaus, C. Genzel, K. M. Ryan and T. Unold, Nat. Commun., 2014, 5, 3133 CAS.
- H.-C. Liao, M.-H. Jao, J.-J. Shyue, Y.-F. Chen and W.-F. Su, J. Mater. Chem. A, 2013, 1, 337–341 CAS.
- Z. G. Li, A. L. K. Lui, K. H. Lam, L. F. Xi and Y. M. Lam, Inorg. Chem., 2014, 53, 10874–10880 CrossRef CAS PubMed.
- A. Khare, A. W. Wills, L. M. Ammerman, D. J. Norris and E. S. Aydil, Chem. Commun., 2011, 47, 11721–11723 RSC.
- S. C. Riha, B. A. Parkinson and A. L. Prieto, J. Am. Chem. Soc., 2009, 131, 12054–12055 CrossRef CAS PubMed.
- T. Kameyama, T. Osaki, K.-I. Okazaki, T. Shibayama, A. Kudo, S. Kuwabata and T. Torimoto, J. Mater. Chem., 2010, 20, 5319–5324 RSC.
- A. Shavel, D. Cadavid, M. Ibáñez, A. Carrete and A. Cabot, J. Am. Chem. Soc., 2012, 134, 1438–1441 CrossRef CAS PubMed.
- M. Li, W.-H. Zhou, J. Guo, Y.-L. Zhou, Z.-L. Hou, J. Jiao, Z.-J. Zhou, Z.-L. Du and S.-X. Wu, J. Phys. Chem. C, 2012, 116, 26507–26516 CAS.
- H. H. Ye, A. W. Tang, L. M. Huang, Y. Wang, C. Yang, Y. B. Hou, H. S. Peng, F. J. Zhang and F. Teng, Langmuir, 2013, 29, 8728–8735 CrossRef CAS PubMed.
- F. Huang, X. L. Wang, J. Xu, D. Q. Chen and Y. S. Wang, J. Mater. Chem., 2012, 22, 22614–22618 RSC.
- P. A. Fernandes, P. M. P. Salomé and A. F. Cunha, J. Alloys Compd., 2011, 509, 7600–7606 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |