Ribbon-like and spontaneously folded structures of tungsten oxide nanofibers fabricated via electrospinning
Abstract
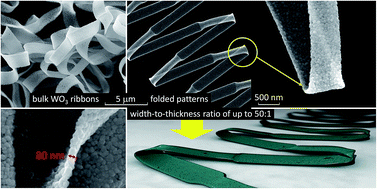
Tungsten oxide (WO3) nanofibers with shapes ranging from cylindrical to ribbon-like were prepared by annealing electrospun polyvinylpyrrolidone/ammonium metatungstate (PVP/AMT) fibers. Formation of periodically folded “zigzag” patterns in PVP/AMT and WO3 ribbon-like fibers was observed for the first time at the initial stage of the electrospinning process on the surface of a stationary substrate. Among methods tested (capillary needle, needleless dc- and ac-electrospinning), only the capillary needle dc-electrospinning process was effective in producing ribbon-like fiber structures. Annealing of such PVP/AMT fibers at 500 °C in air led to the formation of 80 ± 10 nm thick WO3 ribbons with a width-to-thickness ratio of up to 50 : 1. Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), X-ray diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS), and Raman spectroscopy were used to analyze the material. Analyses revealed that regardless the fiber's shape, the annealed oxide fibers were polycrystalline with a grain size of 60 ± 30 nm and consisted of the monoclinic phase of WO3. When compared to cylindrical fibers, the ribbon-like WO3 nanofibers exhibited higher porosity but lower mechanical strength with increased width of the ribbon-like structure.

 Please wait while we load your content...
Please wait while we load your content...