Synthesis of an activated carbon based urea formaldehyde resin and its adsorption and recognition performance towards Fe(III)
Ou Guo-Li,
Gao Jian-Feng,
Hu Tuo-Ping*,
An Fu-Qiang*,
Wang Yu,
Wang Yan-Jun and
Zhang Dong
Chemical Department, School of Science, North University of China, Taiyuan 030051, People’s Republic of China. E-mail: anfuqiang@nuc.edu.cn; 191721629@qq.com; gaojianfeng@126.com; 2211274449@qq.com; 519967452@qq.com; 1123866517@qq.com; 583011084@qq.com; Fax: +86-351-3922118; Tel: +86-351-3923197, +86-351-3921414
First published on 13th August 2015
Abstract
Rare earths are very important strategic resources. However, impurities, such as Fe(III), have great adverse impacts on the properties of rare earth materials. Therefore, the efficient and easy removal of non-rare earth impurities from rare earths is extremely important. A novel activated carbon, ACUF700, was synthesized using a homemade urea formaldehyde resin carbonized at 700 °C. The ACUF700 was characterized using surface area analyzer, FT-IR, elemental analysis, and SEM methods. The adsorption and recognition properties of ACUF700 towards Fe(III) ions were studied. The BET special surface area of ACUF700 was 702.3 cm3 g−1, and the average pore diameter was 2.044 nm. ACUF700 possesses strong adsorption affinity and excellent recognition selectivity for Fe(III). The adsorption capacity could reach 12.8 mg g−1, and the relative selectivity coefficient relative to La(III) was 28.0. Besides, ACUF700 was easily regenerated using a diluted hydrochloric acid solution as the eluent, and ACUF700 possesses good reusability.
1. Introduction
Rare earth elements (REEs) are composed of scandium, yttrium, and the lanthanide metallic elements. As some of the most important strategic and critical mineral resources, REEs have a significant role in luminescence, electronics, magnetism, catalysis, metallurgy, and the ceramic industry.1,2 For high technology materials, non-rare earth impurities, such as Fe(III), have great adverse impacts on the properties of rare earth materials.3 The removal of non-rare earth impurities gets more and more attention. Therefore, the efficient and easy removal of non-rare earth impurities from high purity rare earths is extremely important. Researchers have carried out some related studies in the last century.4,5 Solvent extraction methods and extraction-elution resin (solvent impregnated resin) methods are mainly used. However, some shortcomings, such as a low separating efficiency, are concomitant. Therefore, researching a new effective separation material is important for economic and social benefits.Porous carbons have been widely used as adsorbents,6,7 catalyst supports,8 and electrode materials9 due to their developed pore structure, high-specific surface area and easy surface modifications. The pore structure and surface chemical property of a porous carbon determine its application properties.10–12 Direct preparation using nitrogen-containing compounds as precursors is an effective route to introduce nitrogen into a carbon matrix.13,14
Urea formaldehyde resins (UF resins) have been widely used in adhesives, finishes, medium density fiberboard (MDF), and molded objects. Taking into account their high nitrogen content, UF resins can be used as raw materials for the fabrication of nitrogen-containing activated carbons.15–18
In this study, activated carbon was synthesized using UF resins as raw materials. The pore structure and surface chemical groups of the resultant porous carbon were characterized, and its adsorption and recognition properties for Fe(III) were investigated.
2. Experimental
2.1 Preparation and characterization of ACUF700
To prepare the UF resin, 18 g of NaHCO3, 35 ml of a formaldehyde aqueous solution (37%) and 15 g of urea were mixed in a beaker. The mixture was heated to 95 °C for 5 h. The resultant solid resins were placed in a vacuum oven for 24 h at 80 °C to ensure complete dryness. The powdered resin was carbonized in a charcoal furnace at 700 °C for 2 h with a heating rate of 2 °C min−1 and N2 flow of 50 ml min−1. Finally, the resultant samples were washed with distilled water until the pH was neutral and dried at 80 °C for 24 h.The nitrogen adsorption–desorption isotherm was measured with a surface area analyzer (Beijing JWGB BF-JW132F) with nitrogen absorption at 77 K using the Brunauer–Emmett–Teller (BET) method. Before the measurements, the sample was degassed at 180 °C under vacuum for more than 3 h. The surface area was calculated using the BET method from the adsorption branch in the relative pressure range (P/P0) of 0.005 to 0.1. The SEM images were obtained on a S-4800 Field Emission-Scanning Electron Microscope (Hitachi, Japan) operated at 1 kV. Fourier transform infrared (FTIR) spectra of the samples were measured on a Nicolet FT-IR 4800s (Shimadzu, Japan) spectrometer using the conventional KBr pellet method. The element content was measured with a Vario EL elemental analyzer (Elementar, Germany).
2.2 Batch adsorption of the activated carbon towards Fe(III)
 | (1) |
Several similar conical flasks were prepared and about 0.05 g of ACUF700 was introduced into each flask directly. 25 ml of Fe(III) aqueous solutions with different concentrations (C0) and a same pH of 2 were then added into each conical flask. The conical flasks were placed in a shaker at a presettled temperature. After the adsorption reached equilibrium, the equilibrium concentrations (Ce) of the Fe(III) solutions in the different flasks were determined using an inductively coupled plasma emission spectrometer. The equilibrium adsorption capacities (Qe) were calculated according to eqn (1).
 | (2) |
The selectivity coefficient (k) of ACUF700 for Fe(III) with respect to the competitor species La(III) can be obtained using eqn (3).
 | (3) |
2.3 Dynamic adsorption experiment
2.0 g of ACUF700 was filled in a glass column with 10 ml of the bed volume. The mixture solution of Fe(III) and La(III) with the initial concentrations of 10 mg L−1 and 100 mg L−1, respectively, and a pH of 2, was allowed to flow gradually through the column at a rate of 5 BV h−1. 1 BV of the effluent was collected and the concentration was determined. Then the dynamic adsorption curve was plotted.2.4 Repeated use experiment
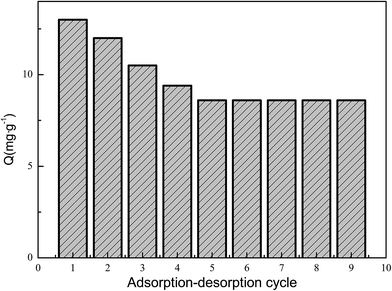
Reusability is an important factor for a good adsorption material. Desorption of the adsorbed Fe(III) on ACUF700 was also studied through a batch experiment. 5 ml of a hydrochloric acid solution with a concentration of 2 mol L−1 was used as the eluent for 0.2 g of adsorbent, and the desorption time was 30 min. In order to test the reusability of ACUF700, the Fe(III) adsorption–desorption procedure was repeated nine times.3. Results and discussion
3.1 Characterization of ACUF700
The Fourier transformed infrared (FTIR) spectra of UF and ACUF700 are shown in Fig. 1. As for the UF resin synthesized here, the band assignment is in good agreement with literature reports.19 The band between 3200 and 3500 cm−1 was attributed to NH stretching vibrations, and the band between 1500 and 1600 cm−1 was attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations. Three bands at 1080, 1039 and 875 cm−1 were assigned to the asymmetric stretching vibration of the ether, C–O stretch of C–OH, and cyclic ether linkages. ACUF700 exhibits broader and overlapping bands due to the strong IR absorption of carbon and the complex rearranged structure. It can be seen in the spectrum of ACUF700 that ACUF700 still retains the amine functional group after carbonization.
C stretching vibrations. Three bands at 1080, 1039 and 875 cm−1 were assigned to the asymmetric stretching vibration of the ether, C–O stretch of C–OH, and cyclic ether linkages. ACUF700 exhibits broader and overlapping bands due to the strong IR absorption of carbon and the complex rearranged structure. It can be seen in the spectrum of ACUF700 that ACUF700 still retains the amine functional group after carbonization.
The morphologies of ACUF700 were characterized using scanning electron microscopy at different magnifications and are shown in Fig. 2.
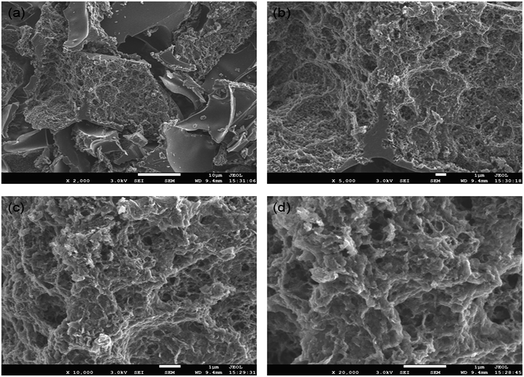 | ||
Fig. 2 SEM images of ACUF700 with different magnifications. (a) x2000; (b) x5000; (c) x10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; (d) x20 000; (d) x20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. 000. | ||
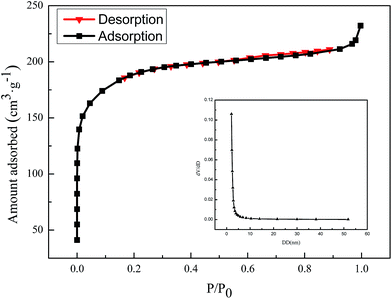
The developed pore structure was easily observed. The relative data are listed in Table 1.
| Sample | Elemental analysis (wt%) | Structure parameters | Yield (%) | |||||
|---|---|---|---|---|---|---|---|---|
| C | N | H | SBET (m2 g−1) | d (nm) | Vmicro (cm3 g−1) | Vtotal (cm3 g−1) | ||
| ACUF400 | 55.46 | 24.54 | 4.26 | 3 | 20.49 | 0.001 | 0.015 | 7.73 |
| ACUF700 | 53.42 | 15.11 | 2.68 | 702 | 2.04 | 0.267 | 0.359 | 4.22 |
| ACUF800 | 67.80 | 9.98 | 2.41 | 1920 | 2.77 | 0.229 | 1.328 | 1.21 |
The surface properties of the samples were measured using the N2 adsorption–desorption isotherms (Fig. 3). The chemical composition and pore structure parameters of ACUF700 are listed in Table 1.
ACUF700 exhibited type I isotherms, and the pore size distribution is narrow.
3.2 Adsorption properties of ACUF700 towards metal ions
The adsorption rates of ACUF700 toward the Fe(III) and La(III) ions were vary fast, the adsorption reached equilibrium within 80 min and 100 min, respectively. It can be seen that the saturated adsorption capacity of ACUF700 for Fe(III) and La(III) is 12.8 mg g−1 and 10.3 mg g−1, respectively. It implies that ACUF700 possesses a very strong adsorption ability for the Fe(III) and La(III) ions.
 | (4) |
The data of Fe(III) and La(III) in Fig. 5 were regressed linearly according to eqn (4) and Table 2 was obtained. The data in Table 2 satisfactorily fit the Langmuir equation, and this clearly indicates that the Fe(III) and La(III) are adsorbed on ACUF700 as monomolecular layers.
 | ||
| Fig. 5 Adsorption isotherms of ACUF700 for Fe(III) and La(III). Temperature: 25 °C; pH = 2; adsorption time: 12 h. | ||
| Ion | Qm | k | R2 | Linear equation |
|---|---|---|---|---|
| Fe3+ | 29.66 | 0.02395 | 0.9974 | Y = 1.4077 + 0.03371X |
| La3+ | 22.71 | 0.01136 | 0.9985 | Y = 3.8745 + 0.04403X |
| Adsorbent | Kd (L g−1) | k | |
|---|---|---|---|
| Fe(III) | La(III) | ||
| ACUF700 | 2.80 | 0.10 | 28.0 |
It can be seen that ACUF700 has high selectivity for Fe(III). This suggests that the adsorption recognition of ACUF700 for Fe(III) is very strong.
3.3 Column adsorption characteristics of ACUF700 towards Fe(III)
 | ||
| Fig. 6 Dynamic adsorption curve of ACUF700 towards the mixture of Fe(III) and La(III). Temperature: 25 °C; pH = 2. | ||
The leaking bed volume for Fe(III) is 120 BV and for La(III) is 18 BV. In addition, the leaking concentration of Fe(III) is lower than 5 mg L−1 before 100 BV. This indicated that Fe(III) can be better recognized by ACUF700, and that ACUF700 can selectively remove Fe(III) from the La(III) solution.
3.4 Desorption and reusability
When hydrochloric acid was used as the eluent, the interaction between Fe(III) and ACUF700 is disrupted and subsequently Fe(III) ions are released into the desorption medium. In order to show the reusability of ACUF700, the adsorption–desorption cycle was repeated 9 times by using the same carbon material. The adsorption–desorption cycle of Fe(III) on ACUF700 is shown in Fig. 7. The result clearly shows that ACUF700 could be used repeatedly without significantly losing its binding ability.4. Conclusions
In this paper, the high-performance activated carbon ACUF700 was synthesized successfully using a homemade urea formaldehyde resin carbonized at 700 °C. The BET special surface area reached 702.3 m2 g−1. The average pore size was 2.044 nm. ACUF700 possesses strong adsorption affinity, specific recognition ability, and excellent selectivity for Fe(III). The adsorption capacity could reach 12.8 mg g−1, and the relative selectivity coefficient relative to La(III) is 28.0. Besides, ACUF700 was easily regenerated using diluted hydrochloric acid solution as the eluent, and ACUF700 possesses good reusability.Acknowledgements
The authors gratefully acknowledge the financial support for this work by the International Science & Technology Cooperation Program of China (No. 2011DFA51980), Shanxi Science & Technology Cooperation Program of China (No. 2015081043), and Science Foundation of Shanxi Province (No. 20130321022-02, 2013021012-3, 20140313002-4).References
- P. Maestro and D. Huguenin, Industrial applications of rare earths: which way for the end of the century, J. Alloys Compd., 1995, 225, 520–528 CrossRef CAS.
- H. Paudyal, B. Pangeni and K. Inoue, et al., Adsorptive removal of fluoride from aqueous solution using orange waste loaded with multi-valent metal ions, J. Hazard. Mater., 2011, 192, 676–682 CrossRef CAS PubMed.
- W. S. Wang, Y. B. Li and B. J. Gao, et al., Effective removal of Fe(II) impurity from rare earth solution using surface imprinted polymer, Chem. Eng. Res. Des., 2013, 91, 2759–2764 CrossRef CAS PubMed.
- J. T. Jia, C. S. Liao and C. H. Yan, et al., On-line solvent extracting Ca from La in La/Ca separation process, Rare Earths, 1999, 20, 12–15 Search PubMed.
- G. B. Li, Y. F. Zhou and J. R. Li, et al., Preparation of high purity lanthana, Rare Met., 1984, 6, 56–58 Search PubMed.
- L. Mouni, D. Merabet and A. Bouzaza, et al., Adsorption of Pb(II) from aqueous solutions using activated carbon developed from Apricot stone, Desalination, 2011, 276, 148–153 CrossRef CAS PubMed.
- V. K. Gupta, A. Rastogi and A. NayaK, Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material, J. Colloid Interface Sci., 2010, 342, 135–141 CrossRef CAS PubMed.
- E. Antolini, Carbon supports for low-temperature fuel cell catalysts, Appl. Catal., B, 2009, 88, 1–24 CrossRef CAS PubMed.
- Z. J. Fan, J. Yan and L. J. Zhi, et al., A Three-Dimensional Carbon Nanotube/Graphene Sandwich and Its Application as Electrode in Supercapacitors, Adv. Mater., 2010, 22, 3723–3727 CrossRef CAS PubMed.
- J. Pyun, Graphene Oxide as Catalyst: Application of Carbon Materials beyond Nanotechnology, Angew. Chem., Int. Ed., 2009, 50, 46–48 CrossRef PubMed.
- S. Z. Mohammadi, M. A. Karimi and D. Afzali, et al., Removal of Pb(II) from aqueous solutions using activated carbon from Sea-buckthorn stones by chemical activation, Desalination, 2010, 262, 86–93 CrossRef CAS PubMed.
- G. R. Li, F. Wang and Q. W. Jiang, et al., Carbon Nanotubes with Titanium Nitride as a Low-Cost Counter-Electrode Material for Dye-Sensitized Solar Cells, Angew. Chem., Int. Ed., 2010, 49, 3653–3656 CrossRef CAS PubMed.
- D. L. Wang, M. M. Chen and C. Y. Wang, et al., Synthesis of carbon microspheres from urea formaldehyde resin, Mater. Lett., 2011, 65, 1069–1072 CrossRef CAS PubMed.
- X. L. Cai, B. Riedl and H. Wan, et al., A study on the curing and viscoelastic characteristics of melamine-urea-formaldehyde resin in the presence of aluminium silicate nanoclays, Composites, Part A, 2010, 41, 604–611 CrossRef PubMed.
- J. H. Yu, M. Y. Guo and F. Muhammad, et al., One-pot synthesis of highly ordered nitrogen-containing mesoporous carbon with resorcinol-urea-formaldehyde resin for CO2 capture, Carbon, 2014, 69, 502–514 CrossRef CAS PubMed.
- Z. Liu, Z. Y. Du and H. Song, et al., The fabrication of porous N-doped carbon from widely available urea formaldehyde resin for carbon dioxide adsorption, J. Colloid Interface Sci., 2014, 416, 124–132 CrossRef CAS PubMed.
- M. J. Xie, Y. F. Xia and J. Y. Liang, et al., Ordered nitrogen doped mesoporous carbon assembled under aqueous acidic conditions and its electrochemical capacitive properties, Microporous Mesoporous Mater., 2014, 197, 237–243 CrossRef CAS PubMed.
- X. Y. Chen, C. Chen and Z. J. Zhang, et al., Nitrogen-doped porous carbon for supercapacitor with long-term electrochemical stability, J. Power Sources, 2013, 230, 50–58 CrossRef CAS PubMed.
- G. Myers, Investigation of urea-formaldehyde polymer cure by infrared, J. Appl. Polym. Sci., 1981, 26, 747–764 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |