Crystal structures and properties of nitrogen oxides under high pressure
Youchun Wang,
Fubo Tian,
Yunzhou Lv,
Da Li ,
Defang Duan,
Xiaojing Sha,
Yunxian Liu,
Zhonglong Zhao,
Ting Yang,
Bingbing Liu and
Tian Cui*
,
Defang Duan,
Xiaojing Sha,
Yunxian Liu,
Zhonglong Zhao,
Ting Yang,
Bingbing Liu and
Tian Cui*
State Key Laboratory of Superhard Materials, College of Physics, Jilin University, Changchun 130012, People's Republic of China. E-mail: cuitian@jlu.edu.cn
First published on 27th November 2015
Abstract
First-principles calculations were performed to investigate the structural, electronic, and elastic properties of N2O4 and N2O5. Two new phases, namely, P42212 N2O4 and P21/m N2O5, are determined under high pressure through an ab initio evolutionary algorithm. For N2O4, the Im3 phase transforms to the P42212 structure at 35 GPa. The pressure–volume curves of N2O4 and N2O5 show that this transition has a first-order nature. The calculated phonon dispersion and elastic constants of Im3 N2O4, P42212 N2O4, and P21/m N2O5 demonstrate that the dynamic and mechanical stable pressure ranges are 2–35, 35–80, and 29–120 GPa, respectively. The electronic properties and projected density of states imply that these three structures are insulators. Furthermore, the N–N and N–O bond length of nitrogen oxides under high pressure are discussed.
1. Introduction
Various pressure-induced transformations, such as structural and electronic phase transitions, chemical reactions, and bonding patterns, in atomic and molecular systems have been studied.1,2 Nitrogen oxides are a group of interest because they are ideal energy storage materials and exhibit complex behavior under high pressure. On the other hand, the biological and environmental chemistry properties of nitrogen oxides and their structurally characteristics are fascinating. Most special is N2O4, which is a planar dimer of NO2 with a longer N–N bond than the conventional N–N single bond.3 The behavior of nitrogen oxides under high pressure has gained increased research attention over the last few decades.Kvick et al.3 studied the cubic phase of planar N2O4 molecules (space group Im3) obtained from condensed gas and found that the phase remains stable at temperatures as low as 20 K. Agnew et al.4 reported that planar and non-cubic β-N2O4 can be formed through laser irradiation of cubic α-N2O4. At a pressure range of 1.5–3 GPa, β-N2O4, which is regarded as metastable, exhibits a reversible phase transition to ionic NO+NO3−, whereas α-N2O4 is stable to at least 7.6 GPa.4 Previous studies elucidated the synthesis of aragonite-type and possible non-centrosymmetric ionic solid NO+NO3− from N2O at high pressures and temperatures.5 Meng et al.6 applied hard X-ray photon radiation to induce dissociation of N2 and O2 molecules and generate ionic NO2+NO3− at 0.5 GPa. At 1.7 GPa, these molecules subsequently decompose into ionic NO+NO3−, whose structure was determined as layer monoclinic P21/m.
Sihachakr and Loubeyre7 found that the ionic compound synthesized from N2/O2 mixtures with 66% O2 exhibits a Pmmm structure at 5.5 GPa, this structure differs from that obtained from the N2O system for the topochemical effect. Song et al.8 studied N2O4 through Raman, synchrotron infrared, and X-ray diffraction measurements and observed a transformation from molecular to ionic structure under high P–T conditions. Kuznetsov et al. and Meng et al. presented different views about transformation of NO+NO3− compound to NO2+NO3− crystal at temperatures higher than ambient and pressure lower than 9 GPa. A series of transformations were demonstrated (NO+NO3− → NO2+NO3− → N2O4), and the spatial group of NO2+NO3− was reported as hexagonal P63/mmc.9 Recently, a new orthorhombic Pna21 phase of NO+NO3− has been reported by Xiao et al.10 through theoretical studies on N2O, the obtained phase is more stable than the monoclinic phase.
To the best of our knowledge, although several experimental studies are available, a few theoretical investigations were performed. Theoretical and experimental investigations about the structure of nitrogen oxides under high pressure have been conducted, however, their structures and mechanical properties under extremely high pressures have not been described. In this work, the thermodynamical and mechanical stability of nitrogen oxides are discussed.
2. Computational methods
In this paper, the evolutionary algorithm11–16 which has been designed to search for the structure with the lowest free energy at high P–T condition is used to search for candidate high pressure structure. The evolutionary algorithm is performed with the USPEX code. Our first-principle calculations on N2O4 and N2O5 are performed with the Cambridge serial total energy package (CASTEP)17,18 code using plane-wave pseudopotential method19 and employing the generalized gradient approximation functional (GGA)20 of Perdew–Burke–Ernzerhof (PBE)21 for the exchange–correlation energy. The energy cutoff was set to 960 eV for the total energy calculations on account of convergence tests. The Monkhorst–Pack K-meshes22 were used with the resolution of 2π × 0.03 Å−1 for Brillouin zone (BZ) sampling to ensure that all the enthalpy calculations were well converged to less than 0.05 meV per atom. The structure relaxation and self-consistent calculations of lattice constants and atomic position are carried out by using the Brodyden–Fletcher–Goldfarb–Shaano (BFGS) method.23 The phonon calculations are carried out using a supercell approach24 combined with linear response theory.3. Results and discussion
3.1. Predicting structures and stability of nitrogen oxides under high pressure
Simulations of an N/O system were performed to predict the structures of the compositions of N2O4 and N2O5 at pressures lower than 120 GPa. Different types of relaxed structures were found. In accordance with the simulations, P42212 N2O4 (2 f.u. per cell) at 60 GPa and P21/m N2O5 (2 f.u. per cell) at 60 GPa are presented in Fig. 1. The Im3 N2O4 at 20 GPa originated from experimental results at low pressure3 and is presented in Fig. 1. For Im3 N2O4, which was obtained from a previous study,3 one nitrogen atom is connected to two oxygen atoms and exists in the form of an NO2 dimer, which is on the same plane. For P42212 N2O4, the two nitrogen atoms are contiguous and each nitrogen atom is connected to two oxygen atoms. Interestingly, one NO2 unit appears to spin, thereby bringing two NO2 units out of the same plane. For P21/m N2O5, one nitrogen atom is linked with three oxygen atoms, with an oxygen atom as the center, and forms a symmetrical structure, which is apparently a layer structure. All nitrogen atoms, as well as all oxygen atoms for the last two phases, are in the same plane.The structural thermodynamical stability was systematically studied. The formation enthalpy were calculated on new nitrogen oxide structures, namely, P42212 N2O4 (2 f.u. per cell), P1 N2O4 (1 f.u. per cell), P1 N2O5 (1 f.u. per cell), and P21/m N2O5 (2 f.u. per cell), and known structures, such as Im3 (3 f.u. per cell, ref. 3), P21/m (2 f.u. per cell, ref. 7), and Pna21 (4 f.u. per cell, ref. 10) for N2O4, as well as P63/mmc (2 f.u. per cell, ref. 9) for N2O5. The calculated enthalpy difference curves for the new structures relative to Im3 N2O4 and P63/mmc N2O5 as a function of pressure are shown in Fig. 2. From Fig. 2(a), P42212 N2O4 is apparently more stable than P1 N2O4 at high pressure and P21/m N2O5 exhibits lower enthalpy than other structures within the whole pressure range presented in Fig. 2(b). Meanwhile, Im3 N2O4 transforms into the P42212 structure at 35 GPa. The calculated pressure–volume data is plotted in Fig. 2(c), which demonstrates that the volume decreases discontinuously about 2.5% with increasing pressure, thereby confirming that the phase transition is a first-order nature.
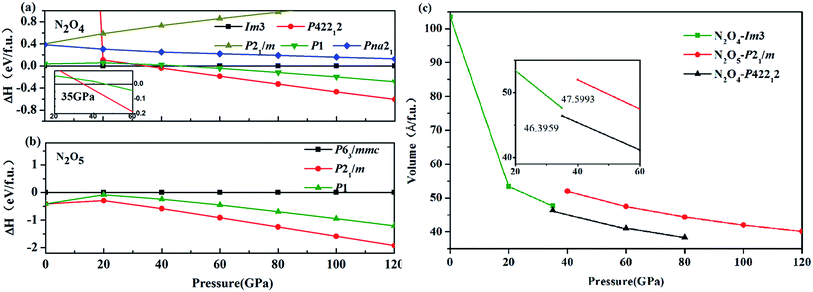 | ||
| Fig. 2 Thermal stability of nitrogen oxides. (a) and (b) Calculated enthalpy difference of nitrogen oxides in the pressure from 2 to 120 GPa. (c) The calculated P–V relationship for N2O4 and N2O5. | ||
The plot of the thermodynamic convex hull shown in Fig. 3(a) indicates the thermodynamical stability of nitrogen oxides. N2O4 appears first at 0 GPa, N2O5 starts to occur under compression at 40 GPa, and both of them coexist between 40 and 80 GPa. At pressure higher than 80 GPa, the enthalpy difference changes make the formation of N2O4 prohibited. This finding indicates that nitrogen content decreases with increasing pressure, that is, energy storage is reduced. Formation enthalpies were calculated and are shown in Fig. 3(b) to confirm the pressure range of N2O4 and N2O5. Accordingly, N2O4 adopts the Im3 phase at pressure ranging from 0 to 35 GPa and the P42212 structure stabilizes at 80 GPa. In addition, P21/m N2O5 is predominant at pressure ranging from 29 GPa to 120 GPa.
 | ||
| Fig. 3 (a) Convex hull curve of nitrogen oxides. (b) The formation enthalpy of N2O4 and N2O5 at pressure range from 0 to 120 GPa. | ||
Phonon dispersion curves were constructed at 0 K to verify the dynamical stability of Im3 N2O4 P42212 N2O4, and P21/m N2O5 at pressures (selected phonon dispersion as shown in Fig. 4(a)). No imaginary phonon frequency exists in the whole Brillouin zone. This finding confirms the dynamical stability of the three phases. In addition, phonon spectrums of Im3 N2O4 at 0 and 2 GPa at 0 K were calculated, which were not shown here. The phonon dispersion results show that Im3 N2O4 is stable in the pressure range of 2 to 35 GPa.
3.2. Electronic properties of nitrogen oxides
The calculated electronic energy band structure of Im3 N2O4, P42212 N2O4, and P21/m N2O5 along high-symmetry directions in the Brillouin zone at 20, 60, and 60 GPa, respectively, are displayed in Fig. 4(b). The three structures are all insulators, as confirmed based on the underestimation of band gap caused by PBE through generalized gradient approximation.10 The variation trend of the band gap for N2O4 and N2O5 on compression is plotted in Fig. 5, which indicates that the band gap of both nitrogen oxides decrease monotonically with increasing pressure. The band gap of N2O5 is significantly larger than N2O4, but the rate of descent for N2O4 is faster than N2O5.Over the past few years, the bonding properties of nitrogen oxides have been investigated25–33 theoretically and experimentally. In this paper, the optimized structure was obtained through density functional theory using CASTEP code with the GGA of PBE for exchange–correlation energy. Theoretical and experimental results of N2O4 are tabulated in Table 1, in which the distance of N–N and N–O decreases with increasing pressure. Kvick et al.3 studied the N–N and N–O bond length of N2O4 at various temperatures, the results showed that RNO decreases with increasing temperature, whereas RNN is not affected by changes in temperature. Hence, the distance of RNO for Im3 N2O4 at 2 GPa is consistent with the experimental value but RNN is higher than the experimental results and other theoretical values. N2O4 is a dipolymer of NO2 bridged by N–N bond. The weak N–N bond may be explained by the electron correction effects,3 N–N antibonding pπ orbitals,32 and HOMO contributions to the N–N bond.32 For example, electron correction effects is a kind of Coulomb interaction, here the two nitrogen atoms are carrying positive charge, so a strong repulsive interaction is there. That makes the bond length between nitrogen and nitrogen is very long and the bond order is very small, so as to make the weak bonding between the two nitrogen atoms. Moreover, the HOMO of electron is antibonding orbital, this characteristic may decrease the bonding energy in molecule.
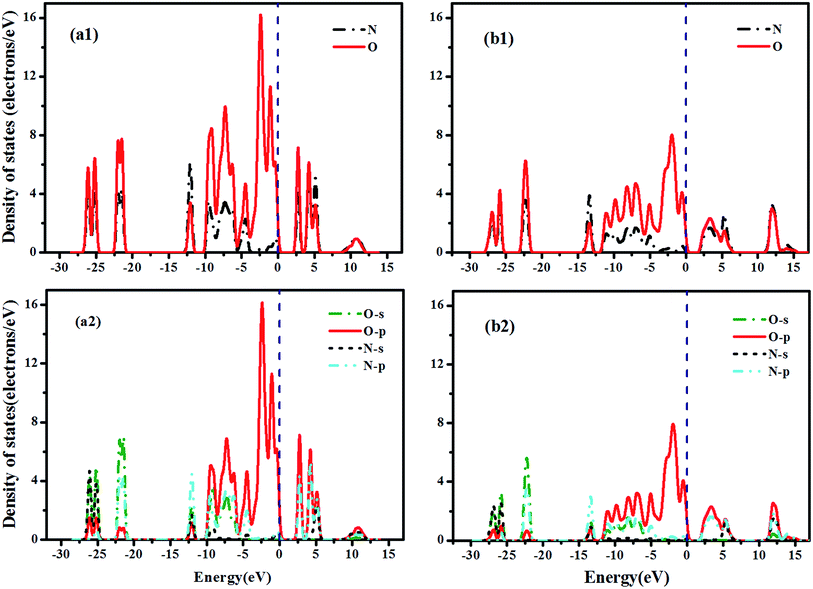
The projected density of states for Im3 N2O4 (20 GPa) and P42212 N2O4 (60 GPa) are shown in Fig. 6. Fig. 6(a1) and (b1) show the intense hybridization of N and O atoms within the whole energy range, except −4 to 0. Moreover, the O atom is the major contributor near the Fermi surface. Fig. 6(a2) and (b2) indicate that O-p and N-p states primarily participate in the bonding. Consequently, we hypothesized that the strong interaction between N and O atoms weakens the N–N bond. A weak N–N bond is considered as an unusual covalent bond, in accordance with the results presented by Chesnut32 and Llusar et al.33 for Im3 N2O4 and P42212 N2O4, respectively. Table 2 presents the three mean values of the N–O bond length of P21/m N2O5 at 60 GPa as well as other theoretical and experimental values, the results reveal that P21/m N2O5 exhibits the C2v structure. The calculated length of three different N–O bonds decreases with increasing pressure. The results we gained are in agreement with the other values in the table, such as the data of ACM, B3P86 and experiment, respectively, which are obtained from the N2O5 molecule. The N–O2 and N–O3 bonds contract smoothly with an overall decrease of 0.51% at 60 GPa. Similarly, the N–O1 distances decrease monotonically with a somewhat larger contraction of 1.68% reflecting the larger compressibility.
3.3. Elastic properties
The elastic constants of Im3 N2O4, P42212 N2O4, and P21/m N2O5 at 20, 60, and 80 GPa, respectively, were calculated to determine the mechanical stability of nitrogen oxides, and the results are listed in Table 3. The elastic constants Cij calculated employing strain-stress relations at finite strains under high pressure. The elastic constants of the three phases satisfy the mechanical stability criteria, hence, these structures are mechanically stable. The well-known Born stability criteria34 for cubic, tetragonal and monoclinic system are cubic phase:35 C11 > 0, C44 > 0, C11 > C12, (C11 + 2C12) > 0.| Im3 | C11 | C44 | C12 | ||||
| 177.92 | 40.94 | 62.86 | |||||
| P42212 | C11 | C33 | C44 | C66 | C12 | C13 | |
| 385.82 | 377.36 | 113.42 | 112.02 | 171.79 | 200.77 | ||
| P21/m | C11 | C22 | C33 | C44 | C55 | C66 | C12 |
| 616.83 | 495.50 | 691.08 | 62.82 | 179.85 | 73.94 | 212.03 | |
| C13 | C15 | C23 | C25 | C35 | C46 | ||
| 198.82 | 34.17 | 208.97 | −1.65 | −44.32 | −2.72 |
Furthermore, the calculated pressure dependencies of normalized lattice constants such as a/a0, b/b0, and c/c0 from 0 to 120 GPa for P21/m N2O5 are illustrated in Fig. 7, where a0, b0, and c0 are the values under ambient condition. The compression of this structure is regarded as anisotropic, with low compressibility along the c-axis and high compressibility along the b-direction, which could be due to the weak force among the layers. The simulation results of elastic constants (Table 3) show that C33 is higher than C11 and C22. Hence, P21/m N2O5 is the most incompressible along the c-axis; this finding is consistent with the normalized lattice constants calculated.
4. Conclusions
In summary, the properties of nitrogen oxides, namely, P42212 N2O4 and P21/m N2O5, under high pressure are revealed through ab initio evolutionary simulations. Im3 N2O4 changes to P42212 N2O4 at 35 GPa, and this phase transition is determined to be a first-order reaction, as evidenced by the discontinuous decrease in volume. The convex hull diagram indicates that P42212 N2O4 and P21/m N2O5 coexist from 29 GPa to 80 GPa. N2O4 adopts Im3, P42212, and P21/m N2O5 within pressure ranges of 2–35, 35–80, and 29–120 GPa, respectively. The phonon dispersion diagrams and elastic constants suggest that the three structures are dynamically and mechanically stable. Meanwhile, the calculated electronic energy bands confirm that these three structures are insulators. We can find that there are no electronic energy bands through the Fermi surface. That confirms both N2O4 and N2O5 are insulator. Meanwhile, the density of states is 0 (electrons per eV) at Fermi level showed in Fig. 6 reveals the same electronic property. The elastic constants calculated also confirm that P21/m N2O5 is the most incompressible along the c-axis, whereas the calculated bond lengths suggest that the N–N bond is an unusual covalent bond.Acknowledgements
This work was supported by the National Basic Research Program of China (No. 2011CB808200), National Natural Science Foundation of China (No. 51572108, 11574109, 11404134, and 11074090), Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1132), National Found for Fostering Talents of basic Science (No. J1103202), and Specialized Research Fund for the Doctoral Program of Higher Education (20120061120008 and 20110061120007), Parts of calculations were performed in the High Performance Computing Center (HPCC) of Jilin University.References
- R. J. Hemley, Annu. Rev. Phys. Chem., 2000, 51, 763–800 CrossRef CAS PubMed.
- J. Sun, D. D. Klug, C. J. Pickard and R. J. Needs, Phys. Rev. Lett., 2011, 106, 145502 CrossRef PubMed.
- A. Kvick, R. K. McMullan and M. D. Newton, J. Chem. Phys., 1982, 76, 3754–3761 CrossRef CAS.
- S. F. Agnew, B. I. Swanson, L. H. Jones, R. L. Mills and D. Schiferl, J. Phys. Chem., 1983, 87, 5065–5068 CrossRef CAS.
- S. Maddury, A. Madduri, A. F. Goncharov, O. Tschauner, P. F. McMillan, H. R. Mao and R. J. Hemley, Phys. Rev. Lett., 2011, 87, 135504 Search PubMed.
- Y. Meng, R. B. Von Dreele, B. H. Toby, P. Chow, M. Y. Hu, G. Y. Shen and H. K. Mao, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 214107 CrossRef.
- D. Sihachakr and P. Loubeyre, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 064113 CrossRef.
- Y. Song, R. J. Hemley, H. K. Mao, Z. X. Liu and D. R. Herschbach, Chem. Phys. Lett., 2003, 382, 686–692 CrossRef CAS.
- Y. Kuznetsov, L. Dubrovinsky, A. Kurnosov, M. M. Lucchese, W. Crichton and C. A. Achete, Adv. Phys. Chem., 2009, 2009, 180784 Search PubMed.
- H. Xiao, Q. An, W. A. Goddard, W. G. Liu and S. V. Zybin, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 5321–5325 CrossRef CAS PubMed.
- A. R. Oganov and C. W. Glass, J. Chem. Phys., 2006, 124, 244704–244715 CrossRef PubMed.
- C. W. Glass, A. R. Oganov and N. Hansen, Comput. Phys. Commun., 2006, 175, 713–720 CrossRef CAS.
- R. Oganov, C. W. Glass and S. Ono, Earth Planet. Sci. Lett., 2006, 241, 95–103 CrossRef.
- O. Lyakhov, A. R. Oganov and M. Valle, Comput. Phys. Commun., 2010, 181, 1623–1632 CrossRef.
- A. R. Oganov, A. O Lyakhov and M. Vallen, Acc. Chem. Res., 2011, 44, 227–237 CrossRef CAS PubMed.
- A. O. Lyakhov, A. R. Oganov, H. T. Stokes and Q. Zhu, Comput. Phys. Commun., 2013, 184, 1172–1182 CrossRef CAS.
- M. D. Segall, P. J. D. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 2005, 220, 567–570 CAS.
- D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 7892 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- B. Hammer, L. B. Hansen and J. K. Norskov, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 7413 CrossRef.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- R. H. Byrd and J. Nocedal, SIAM J. Numer. Anal., 1989, 26, 727–739 CrossRef.
- K. Parlinski, Z. Li and Y. Kawazoe, Phys. Rev. Lett., 1997, 78, 4063–4066 CrossRef CAS.
- B. W. McClemlland, G. Gundersen and K. J. Hedberg, J. Chem. Phys., 1972, 56, 4541 CrossRef.
- B. W. McClemlland, L. Hedberg, K. Hedberg and K. Hagen, J. Am. Chem. Soc., 1983, 105, 3789–3793 CrossRef.
- A. Stirling, L. Papal, J. Mink and D. R. Salahub, J. Chem. Phys., 1994, 100, 2910 CrossRef CAS.
- H. Munakata, T. Kakumoto and J. Baker, J. Mol. Struct., 1997, 391, 231–240 CrossRef CAS.
- S. S. Wesolowski, J. T. Fermann, T. D. Crawford and H. F. Schaefer, J. Chem. Phys., 1997, 106, 7178 CrossRef CAS.
- K. B. Borisenko, M. Kolonits, B. Rozsondai and I. Hargittai, J. Mol. Struct., 1997, 413, 121–131 Search PubMed.
- P. Aplincourt, F. Bohr and M. F. Ruiz-Lopez, J. Mol. Struct., 1998, 426, 95–104 CrossRef CAS.
- D. B. Chesnut and A. L. Crumbliss, Chem. Phys., 2005, 315, 53–58 CrossRef CAS.
- R. Llusar, A. Beltran, J. Andres, S. Noury and B. Silvi, J. Comput. Chem., 1999, 20, 1517–1526 CrossRef CAS.
- M. Born and K. Huang, Dynamical Theory of Crystal Lattices, Clarendon, Oxford, 1956 Search PubMed.
- Z. J. Wu, E. J. Zhao, H. P. Xiang, X. F. Hao, X. J. Liu and J. Meng, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 054115 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |