Growth habit and optical properties of γ-CuI single crystals via a temperature difference method
Yangyang Lva,
Liwang Yeb,
Zhaojun Zhangb,
Bin-Bin Zhanga,
Zhihuang Xub,
Xinxin Zhuang*b,
Shuhua Yao*a and
Genbo Sub
aNational Laboratory of Solid State Microstructures and Department of Materials Science and Engineering, Nanjing University, Nanjing 210093, PR China. E-mail: shyao@nju.edu.cn
bKey Laboratory of Optoelectronic Materials Chemistry and Physics, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, PR China. E-mail: zxx@fjirsm.ac.cn
First published on 7th August 2015
Abstract
High quality γ-CuI single crystals have been grown using a mild temperature difference method with ammonium halide (NH4X, X = Cl, Br, I) as the co-solvents. The growth habit and optical properties of the crystals obtained using different co-solvents were systematically examined. By changing the type of co-solvent, the crystallites obtained display octahedron or tetrahedron morphology. All the as-grown crystals are of high transmittance (over 70%). Simultaneously, the crystals exhibit a sharp band-edge emission at around 411 nm, indicating that the interband excitonic transition emission occupies a dominant position in the spectrum and the intensity of defect emission is dramatically depressed. The possible mechanisms of how the co-solvent influences the growth habit and luminescence properties of the γ-CuI crystals are discussed. In particular, our results provide valuable clues to improve the luminescence performance of γ-CuI crystals.
1. Introduction
Recently, cubic γ-CuI has attracted steadily growing attention as it is the fastest room temperature inorganic scintillation crystal (ultrafast scintillation decay time of about 90 ps) reported to date.1,2 Moreover, it is also a p-type direct wide band-gap (Eg = 3.1 eV) semiconductor, which has potential application in the field of opto-electrical devices.3 However, the low intensity of the ultrafast luminescence component at room temperature will be a restriction for its application as an ultra-fast scintillator. Therefore, the preparation of γ-CuI single crystals with excellent luminescent characteristics, especially an ultrafast component luminescence, is of primary importance. In addition, research on the luminescence properties of γ-CuI crystals will also promote their application in opto-electrical devices (e.g. LEDs, detectors). In general, the optical properties are highly dependent on the crystal quality, which is sensitive to the growth process. In order to grow high-quality γ-CuI crystal, various approaches have been attempted such as the flux method, sublimation techniques, sol–gel methods, hydrothermal routes and evaporation methods.4–9 However, high quality crystals with great optical properties, such as high transmittance and luminescence emission without the defect band, have been rarely reported. Therefore, it is still a great challenge to explore methods for the growth of γ-CuI single crystals with sufficient size for research and practical applications.The solution growth method is a versatile approach used to grow large optical-grade single crystals with low defect density.10 However, γ-CuI is almost insoluble in water (pKsp = 11.96 at 300 K), therefore a co-solvent is required for the solution growth of γ-CuI. On the basis of our previous studies,11 we concluded that NH4X (X = Cl, Br, I) co-solvents can effectively increase the solubility of γ-CuI in water and be well suited for crystal growth via a low-temperature aqueous solution method. In this study, a home-designed temperature difference method was used for high optical quality γ-CuI crystal growth with NH4X as the co-solvent employing a positive temperature gradient. For the solution method growing crystal, the crystal growth habit of inorganic nano/micro-crystals is also of special interest owing to the unique properties and applications for materials of different morphology.12 Therefore, the correlation between the morphology of the γ-CuI crystal grains and the co-solvents used was investigated. Then, the optical properties of the crystals grown using different co-solvents were studied. The present results give us an insight on the shape controlled synthesis of γ-CuI nano/micro-crystals and the optimization of the luminescence performance of γ-CuI crystals.
2. Experimental
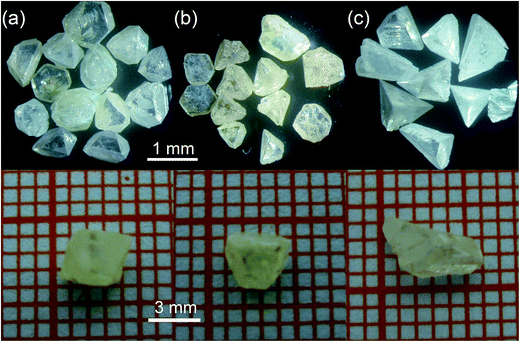
All reagents (A.R.) were purchased from Sinopharm Chemical Reagent Co., Ltd (P. R. China). The γ-CuI seed crystals were obtained from spontaneous crystallization experiments by cooling a saturated solution. The saturated solutions were prepared by dissolving 1.17, 6.65 and 43.91 g of γ-CuI powder in 200 mL of a 6 mol L−1 aqueous solution of NH4X at 60 °C. Copper sheets and liquid paraffin seal were used to prevent the oxidation reaction of the I anions. Several days later, the as-grown γ-CuI crystallites were obtained by slowly cooling the hot-filtered solution. Then, the crystallites were washed with ultra-pure water and dried naturally for the further studies. The crystallites with size nearly 1 mm3 were chosen as seeds and mounted on a circular organic glass bar to grow large single crystals from the respective NH4X aqueous solutions. Crystal growth was performed in a home-designed apparatus. As shown in Fig. 1, the excess polycrystalline γ-CuI nutrient was placed at the bottom of the container (hot zone, 60 °C) to maintain the Cu+ and I− ion concentration for a saturated solution and the seed was placed at the upper half (cold zone, 40 °C) surrounded by the supersaturated solution. The temperature difference between the bottom and the upper part of the solution induced sufficient convection to transport the growth units to the seeds. Then, as the γ-CuI powder was dissolved at the bottom half, the seeds gradually grow into large single crystals. After 30 days, bulk γ-CuI crystals with sizes up to 1 cm3 were obtained using the different co-solvents. To simplify the manuscript, we use γ-CuIa, γ-CuIb and γ-CuIc to represent the crystals grown from the NH4Cl, NH4Br and NH4I solutions, respectively, in the subsequent discussion.X-ray diffraction (XRD) patterns were recorded on a DMAX 2500 powder diffractometer with a graphite monochromatized Cu Kα radiation with 2θ scans from 5° to 65° using a tube voltage of 40 kV and tube current of 40 mA. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Fisher Scientific Co. ESCALAB 250 equipped with a monochromatic Al Kα X-ray radiation source (hν = 1486.6 eV). In the XPS measurements, the binding energy (BE) scale was calibrated against the BE of C 1s at 284.6 eV as an internal standard. Optical transmission spectra were obtained using a SHIMADZU UV-2550 spectrometer with wavelengths ranging from 200 nm to 700 nm. Photoluminescence studies were carried out on an Edinburgh FLS920 fluorescence spectrometer with wavelengths from 350 to 500 nm using an excitation wavelength of 325 nm. All the measurements were performed at room temperature.
3. Results and discussion
3.1 Morphology of the γ-CuI crystallites
As shown in Fig. 2, the crystallites (Fig. 2(a) and (b)) gained from NH4Cl or NH4Br solutions appear octahedral, whereas the crystallites obtained from NH4I solutions appear tetrahedral (Fig. 2(c)). The morphology of γ-CuI crystallite mainly depends on the growth rate ratio of the different crystal facets. Different facets display different co-ordination numbers (CNS) of the ions exposed on it, and the face with fewer CNS has a faster growth rate.13 In our experiment, the exposing ions on the {001} and {111} faces are determined by the co-solvent employed, which decides the state of the Cu+ and I− ions on the rate-determining interface between the crystal and solution. When NH4Br or NH4Cl were used as the co-solvent, the concentrations of I− and Cu+ in the solutions are nearly equal. Thus, the contribution of Cu+ and I− ions to the growth habits of the {001} and {111} faces are equivalent. In addition, the CNS of the determining ions (both Cu+ and I− ions) at the interface of the {001}, (111) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) faces are 2, 3 and 3, respectively. Therefore, the growth rates obey a rule as follows: R {001} > R (111) = R (
) faces are 2, 3 and 3, respectively. Therefore, the growth rates obey a rule as follows: R {001} > R (111) = R (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ). Consequently, the {001} faces disappear easily during the crystal growth process. The obtained γ-CuI crystallites often display an octahedron growth habit. However, as the concentration of Cu+ ions was much less than I− in the solution, the growth habits of the {001} and {111} faces are mainly restricted by the interface characteristics of the Cu+ ions when NH4I solution was chosen as the co-solvent. In addition, the effective CNS of Cu+ ions on the {001}, (111) and (
). Consequently, the {001} faces disappear easily during the crystal growth process. The obtained γ-CuI crystallites often display an octahedron growth habit. However, as the concentration of Cu+ ions was much less than I− in the solution, the growth habits of the {001} and {111} faces are mainly restricted by the interface characteristics of the Cu+ ions when NH4I solution was chosen as the co-solvent. In addition, the effective CNS of Cu+ ions on the {001}, (111) and (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) faces are 2, 1 and 3, respectively. Thus, their growth rates obey the rule as follows: R (111) > R {001} > R (
) faces are 2, 1 and 3, respectively. Thus, their growth rates obey the rule as follows: R (111) > R {001} > R (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), and the (
), and the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) faces always grow into the dominant exposed surface. Consequently, the γ-CuI crystallites obtained often show a tetrahedron morphology.
) faces always grow into the dominant exposed surface. Consequently, the γ-CuI crystallites obtained often show a tetrahedron morphology.
3.2 Structure and composition characterization of the γ-CuI crystals
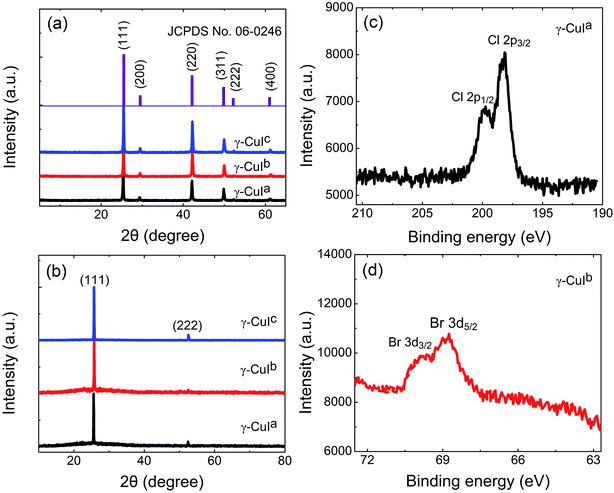
As depicted in Fig. 3(a), all the diffraction powder peaks in the XRD patterns of the crystals prepared using different co-solvents agree with the standard (JCPDS card no. 06-0246, space group: F43m) of γ-CuI. No reflections originating from extra phases were observed, indicating that all the as-grown crystals belong to the γ-phase with a cubic structure. Then, all three as-grown crystal samples were cut along the dominant exposed surface with a thickness of about 1 mm and polished for XRD measurements. The XRD patterns only show the reflections from the {111} faces, confirming that the {111} faces easily appear. In the XPS experiment, a survey scan was obtained to investigate the elements in the crystal. The XPS Cl 2p core level of the γ-CuIa crystal and Br 3d core level of the γ-CuIb crystal are revealed in Fig. 3(c) and (d), respectively. The Cl 2p3/2 and Cl 2p1/2 core levels appear at the binding energies of about 198.8 and 200.4 eV, respectively. In addition, the binding energies of Br 3d5/2 and Br 3d3/2 are centered at around 68.9 and 69.8 eV, respectively. Quantification of the elemental content (at%) of Cl1− or Br1− impurity ions within the two crystals was obtained from the XPS spectra and found to be 1.23% and 2.19%, respectively. The results demonstrate that the γ-CuIa and γ-CuIb crystals contained trace Cl1− and Br1−, respectively. The XPS of the γ-CuIc crystal shows that it is made up of pure γ-CuI crystals without extra impurities. In the following section, the influence of impurities on the optical properties of the as-grown crystals will be discussed.3.3 Optical properties of the γ-CuI crystals
Crystal platelets with a thickness of about 1 mm were cut along the (111) face and polished for optical measurements. The transmittance spectra of the as-grown γ-CuI crystals are plotted in Fig. 4(a). All the crystals show transparent windows in the range of visible light and exhibit a maximum transmittance exceeding 70%, which indicates that the as-grown crystals have a high optical quality. After careful observations, we find that the transmittance of the γ-CuIc crystal was higher than that of the γ-CuIa and γ-CuIb crystals. Moreover, the γ-CuIa, γ-CuIb and γ-CuIc crystals also display sharp cut down edges located at 412, 417 and 411 nm, respectively, which are a characteristic for direct-gap interband transitions.9 The difference was possibly due to the existence of trace Cl1− or Br1− impurities in γ-CuIa and γ-CuIb, respectively.Fig. 4(b) exhibits the photoluminescence spectra of the as-grown γ-CuI crystals at room temperature. An asymmetrical band-edge emission of a two-peak structure was located at around 412 nm in the spectrum of the γ-CuIb crystal, whereas symmetrical emissions at 412 nm and 411 nm were observed in the spectra of the γ-CuIa and γ-CuIc crystals, respectively. It should be mentioned that all the band-edge emissions are sharp and have a strong intensity, indicating the appearance of an interband excitonic transition, which was also evidence for the high quality of the crystal.14 The emission band of the γ-CuIb crystal was fitted with a Gaussian line shape and the results show that there are two luminescent peaks at approximately 411 and 419 nm, respectively. The broad band at 419 nm was the defect emission band and possibly originated from the recombination of the donor–acceptor pair (DAP) emission.15 In general, the emission energy of DAP depends on the power intensity of the excitation light according to previous theory.16 Thus, we tested the emission of the same γ-CuIb crystal at 419 nm under different excitation power of the incident 325 nm laser to speculate if the emission originates from DAP. As shown in Fig. 4(c), P0 is the initial power of excitation light and P0/e0.3 and P0/e represent the power attenuated to 1/e0.3 and 1/e times, respectively. It shows that the peak position of the broad emission band shifts towards a slightly lower energy upon decreasing the power of excitation light. Considering the XPS characterization, as the ion radius of Br1− ions is smaller than I1− ions, trace Br1− impurities exists in the γ-CuIb crystal. Thus, we assume that the existing Br1− ions may act as an acceptor level and the Cu interstices acts as the donor of the DAP emission. The emission at 419 nm may originate from DAP. To confirm the origin, bulk γ-CuBr crystals with sizes up to 5 mm were also grown using the abovementioned temperature difference method from a 3 mol L−1 aqueous solution of HBr. The room temperature photoluminescence spectrum of the γ-CuBr crystal was obtained under the same conditions used for γ-CuIc, as shown in Fig. 4(d). As can be seen, the peak position of the emission of the γ-CuBr crystals was located at 419 nm in the band-edge region (Eg = 2.95 eV (ref. 17)), which was close to the broad emission band of the γ-CuIb crystal. The results indicate that the appearance of a γ-CuBr phase in the as-grown γ-CuIb crystal is another possible reason for the emission at 419 nm.18 The exact origin remains unclear and a more detailed investigation is currently in progress.
4. Conclusions
In this study, we proposed a facile and efficient approach for the growth of high quality γ-CuI single crystals via a temperature difference method using NH4X (X = Cl, Br, I) as the co-solvents. The co-solvents have a remarkable influence both on the morphology and the optical properties of the γ-CuI crystals. The correlation between the crystal growth habit and the co-solvent will make it possible to produce crystallites with a desired morphology. In addition, the as-grown crystals exhibit excellent optical properties with a maximum transmittance exceeding 70% and a sharp band-edge emission. The sharp emission at 411 nm was attributed to the interband excitonic transition. The broad emission band of the as-grown γ-CuIb crystal at 419 nm may originate from DAP emission or γ-CuBr phase in the γ-CuIb crystal. These results provide an important reference for investigating the luminescence mechanism of γ-CuI crystals and improving their luminescence properties, especially their fast component luminescence by doping under mild growth conditions.Acknowledgements
We would like to acknowledge the financial support from the Graduate Innovation Fund of Nanjing University and the open project of the Key Laboratory of Optoelectronic Materials Chemistry and Physics, Chinese Academy of Sciences. Shuhua Yao acknowledges the financial support from the National Natural Science Foundation of China (51472112).References
- S. E. Derenzo, M. J. Weber and M. K. Klintenberg, Nucl. Instrum. Methods Phys. Res., Sect. A, 2002, 486, 214–219 CrossRef CAS.
- I. Tanaka, D. Kim, M. Nakayama and H. Nishimura, J. Lumin., 2000, 87–89, 257–259 CrossRef CAS.
- W. Sekkal and A. Zaoui, Phys. B, 2002, 315, 201–209 CrossRef CAS.
- C. Schwab and A. Goltzené, Prog. Cryst. Growth Charact., 1982, 5, 233–276 CrossRef CAS.
- T. Goto, T. Takahashi and M. Ueta, J. Phys. Soc. Jpn., 1968, 24, 314–327 CrossRef CAS.
- M. Gu, D. X. Wang, Y. T. Huang and R. Zhang, Cryst. Res. Technol., 2004, 39, 1104–1107 CrossRef CAS PubMed.
- D. G. Chen, Y. J. Wang, Z. Lin, J. K. Huang, X. Z. Chen, D. M. Pan and F. Huang, Cryst. Growth Des., 2010, 10, 2057–2060 CAS.
- J. G. Pan, S. Y. Yang, Y. B. Li, L. Han, X. Li and Y. J. Cui, Cryst. Growth Des., 2009, 9, 3825–3827 CAS.
- M. Gu, P. Gao, X. L. Liu, S. M. Huang, B. Liu, C. Ni, R. K. Xu and J. M. Ning, Mater. Res. Bull., 2010, 45, 636–639 CrossRef CAS PubMed.
- G. B. Su, X. X. Zhuang, Y. P. He and G. Z. Zheng, Opt. Mater., 2008, 30, 916–919 CrossRef CAS PubMed.
- Y. Y. Lv, Z. H. Xu, L. W. Ye, Z. J. Zhang, G. B. Su and X. X. Zhuang, CrystEngComm, 2015, 17, 862–867 RSC.
- A. P. Alivisatos, Science, 1996, 271, 933–937 CAS.
- W. J. Li and E. W. Shi, Cryst. Res. Technol., 2002, 37, 1041–1048 CrossRef CAS.
- Y. S. Ma, M. Gu, S. M. Huang, X. L. Liu, B. Liu and C. Ni, Mater. Lett., 2013, 100, 166–169 CrossRef CAS PubMed.
- I. K. Vereshchagin, V. A. Nikitenko and S. G. Stoyukhin, J. Lumin., 1984, 29, 215–221 CrossRef CAS.
- H. L. Liu, M. Gu, R. Zhang, R. K. Xu, G. W. Li and X. P. Ouyang, Acta Phys. Sin., 2006, 55, 6574–6579 CAS.
- Y. Y. Lv, Z. H. Xu, L. W. Ye, G. B. Su and X. X. Zhuang, J. Cryst. Growth, 2014, 402, 337–341 CrossRef CAS PubMed.
- I. Tanaka and M. Nakayama, J. Appl. Phys., 2002, 92, 3511–3516 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |