Electroreduction-based Tb extraction from Tb4O7 on different substrates: understanding Al–Tb alloy formation mechanism in LiCl–KCl melt
Li-Xia Luoab,
Ya-Lan Liub,
Ning Liu*a,
Kui Liub,
Li-Yong Yuanb,
Zhi-Fang Chaib and
Wei-Qun Shi*b
aKey Laboratory of Radiation Physics and Technology, Ministry of Education, Institute of Nuclear Science and Technology, Sichuan University, Chengdu 610064, China. E-mail: nliu720@scu.edu.cn
bLaboratory of Nuclear Energy Chemistry, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China. E-mail: shiwq@ihep.ac.cn
First published on 29th July 2015
Abstract
This work presents the electroreduction of Tb(III) ions, and formation mechanisms of Al–Tb alloys in molten chlorides by applying different types of cathodes: Mo, Al and Al-coated Mo. First, Tb(III) ions were successfully produced by the chlorination of Tb4O7 with AlCl3 in this work. Next, the mechanisms of electrode reactions were determined by various electrochemical techniques, such as cyclic voltammetry (CV), square wave voltammetry (SWV), chronopotentiometry (CP) and open circuit chronopotentiometry (OCP). On the Mo electrode, the reduction of Tb(III) to Tb(0) was determined to be reversible and a one-step process with three electrons exchanged, which was mainly controlled by the mass transport process of linear diffusion of Tb(III) in a chloride melt. In addition, the diffusion coefficient of Tb(III) was calculated to be (2.29 ± 0.01) ×10−5 cm2 s−1 by the Sand equation. According to electrochemical investigations, it was clear that Al–Tb alloy formation was feasible on both solid aluminum and Al-coated molybdenum electrodes. Three redox signals corresponding to the formation and dissolution of different kinds of Al–Tb intermetallic compounds were observed on the Al-coated Mo electrode, whereas only one redox signal was detected on the solid Al electrode. Finally, deposited Al–Tb alloy samples were prepared by potentiostatic electrolysis and characterized by scanning electronic microscopy coupled with energy dispersive spectrum (SEM-EDS) and X-ray diffraction (XRD). It was found that the intermetallic compound Al2Tb was formed by potentiostatic electrolysis at a potential of −1.6 V and at a temperature of 803 K. When the deposition temperature was elevated to 903 K, the intermetallic compound Al3Tb was then obtained by potentiostatic electrolysis.
Introduction
It is well known that a large number of highly radioactive elements such as the long-lived minor actinides (MAs) and fission products (FPs) from spent nuclear fuels are thought of as damaging to human health and the environment.1 Therefore, the minimization of the toxicity of high-level radioactive wastes warrants no delay. The so-called pyro-reprocessing technology, which adopts an electro-refining process in a molten salt bath to recover useful actinides, has been increasingly researched in the past several decades and is deemed to be one of the promising strategies for future spent nuclear fuel management.2,3Under this circumstance, investigations on the electrochemical behaviors of lanthanides (Lns) and actinides (Ans) in various molten salts have been carried out in the past decades.4,5 However, up to now, efficient separation of Ans over Lns is still a challenging topic due to their similar chemical properties. As far as the electro-deposition of Lns and Ans is concerned, it is noted that an aluminum cathode, as an alternative active electrode, can make the reductive potentials of Lns and Ans shift to more positive values, compared to those on an inert electrode, by forming Al–Ln and Al–An alloys. More significantly, the disparity of deposition potentials between Ans and Lns on the Al electrode is much larger than that on the traditional Cd and Bi electrodes,6 which may favorably facilitate the separation of Lns and Ans.7,8 Therefore, the Al cathode has attracted specific attention as a promising cathode for the efficient separation of Ans from Lns.9 Up to now, the deposition of Lns, such as Ho, Eu, Gd, Sm, and Ce, on an Al electrode had been explored.10–14 Taxil et al.15 and Gibilaro et al.16 concluded that the extraction efficiency of Lns could exceed 99% by the co-reduction of a Ln element with Al on an inert electrode.
Nowadays, the electroreduction of actinide oxides as the precursor materials directly added into the molten salt has been paid much attention. This is due to the fact that chlorination of oxide fuels is extremely challenging and often needs the use of strongly toxic and corrosive hydrogen chloride gas, although its reaction kinetics are remarkably slow. Therefore, for the improvement and optimization of the process, it is quite meaningful to study the electrochemistry of Ans and Lns in a chloride molten salt with metal oxides as the starting materials. Additionally, Zhang et al.17 reported that LnxOy and AnxOy could be successfully converted into Ln and An chlorides using AlCl3 as the chlorination agent in the melt. In our group, the electrochemical reduction of oxide precursors, such as Gd2O3, CeO2, Er2O3, Dy2O3 and ThO2, assisted by AlCl3, in a LiCl–KCl melt12,14,18–20 had been systematically investigated.
Up to date, several works have focused on the electrochemical extraction of Tb(III) in molten salt.21–29 For example, the electro-depositions of terbium ions using active electrodes (Ni,28,29 liquid Cd24) and the nucleation-controlled process of Tb(III) with a W electrode27 have been explored. Additionally, the thermodynamic and fluorescence spectroscopic properties of Tb(III) have also been evaluated.24,26,28 Direct electrochemical depositions of Tb–Ni and Tb–Fe intermetallic compounds from terbium oxide in molten CaCl2 were also reported by Qiu et al.21,22 However, to the best of our knowledge, investigations on the electrochemical reduction of Tb(III) ions that originated from the oxide have not been addressed yet, although it is an important measure to achieve an efficient terbium metal extraction from Tb4O7. In this work, we have conducted the electrochemical study of Tb(III) ions on a Mo electrode in a LiCl–KCl eutectic. A combination of transient electrochemical techniques, such as CV, SWV, and OCP, has been applied in order to understand the mechanism of Tb(III) reduction and the formation of Al–Tb alloys on the Al-coated molybdenum and Al plate electrodes in the LiCl–KCl–AlCl3–Tb4O7 system.
Experimental
Chemicals and preparation of electrolytes
Lithium and potassium chloride (anhydrous, AR grade, Sinopharm Chemical Reagent Co. Ltd), terbium oxide (anhydrous) and aluminum chloride (anhydrous) were used for the studies. A mixture of LiCl–KCl with the eutectic composition LiCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KCl = 44.8
KCl = 44.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55.2 wt% was pre-dried under vacuum for more than 72 h at 473 K to remove residual water, and then was melted in a 200 cm3 alumina crucible placed in a cylindrical quartz cell located inside an electric furnace connected by a West 3300 programmable device controlling its temperature. Moreover, all the chemicals were handled in an inert argon atmosphere to avert exposure to O2 and H2O. The O2 and H2O levels were controlled to be less than 2 ppm. In the experiment, the operating temperature was measured by a calibrated nickel–chromium thermocouple kept in a quartz tube, which was inserted into the melt. TbCl3 in the melt was prepared from Tb4O7 and AlCl3 as follows:30,31 in order to reach the maximum chlorination of Tb4O7, excessive AlCl3 powder was directly introduced into the LiCl–KCl eutectic at 787 K and then the un-reacted AlCl3 removed by Ar gas bubbling through the melt along with stirring during the experiment. ICP-AES results of the collected samples taken from the chlorinated salt mixtures indicated that roughly 92% of Tb4O7 had been chloridized.
55.2 wt% was pre-dried under vacuum for more than 72 h at 473 K to remove residual water, and then was melted in a 200 cm3 alumina crucible placed in a cylindrical quartz cell located inside an electric furnace connected by a West 3300 programmable device controlling its temperature. Moreover, all the chemicals were handled in an inert argon atmosphere to avert exposure to O2 and H2O. The O2 and H2O levels were controlled to be less than 2 ppm. In the experiment, the operating temperature was measured by a calibrated nickel–chromium thermocouple kept in a quartz tube, which was inserted into the melt. TbCl3 in the melt was prepared from Tb4O7 and AlCl3 as follows:30,31 in order to reach the maximum chlorination of Tb4O7, excessive AlCl3 powder was directly introduced into the LiCl–KCl eutectic at 787 K and then the un-reacted AlCl3 removed by Ar gas bubbling through the melt along with stirring during the experiment. ICP-AES results of the collected samples taken from the chlorinated salt mixtures indicated that roughly 92% of Tb4O7 had been chloridized.
Electrodes and instrumentation
The counter electrode was a pure graphite rod ϕ 6.0 mm with a large surface area, in order to ensure uniform distribution of current lines. The Ag/AgCl system served as the reference electrode. It consisted of a silver wire ϕ 1.0 mm inserted into a closed Pyrex-glass tube, containing LiCl–KCl eutectic salt together with 1 wt% AgCl. In our experiment, all the potentials were referred to this reference. As working electrodes, molybdenum and aluminum wires of 1.0 mm diameter were used for inert and active electrodes, respectively. The aim is to understand the electrochemical behavior that occurs in this system. In addition, the working electrodes were cleaned by anodic polarization before each measurement. The immersion depth of the electrode in molten salt was recorded to determine the surface area after each measurement. All electrochemical measurements were carried out using Autolab PGSTAT302 (Metrohm Co., Ltd) potentiostat/galvanostat controlled with the Nova 1.9 software package.Preparation and characterization of Al–Tb alloys
Potentiostatic electrolysis was employed to prepare Al–Tb intermetallic compounds using aluminum plates as electrodes in the LiCl–KCl–AlCl3–Tb4O7 melt. The aluminum plates were drawn out and washed with ethanol by ultrasound to remove excessive salt. Afterwards, these samples were dried in a vacuum drying oven and then stored in the glove box before analysis. The cathode deposits were analyzed by X-ray diffraction (XRD, Bruker D8). The cross-section of the deposits was characterized using scanning electron microscopy (SEM, Hitachi S-4800) with energy dispersive spectroscopy (EDS, GENESIS 2000) to obtain their surface morphology and micro composition. Finally, the salt samples were dissolved in a concentrated nitric acid solution, and determined by an inductively coupled plasma atomic emission spectrometer (ICP-AES, IRIS Intrepid II XSP) to confirm the concentration of Tb in the samples.Results and discussion
Chlorination of Tb4O7
Under a low O2 partial pressure, Tb4O7 can thermally decompose32 and the reaction equation can be described as follows:
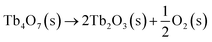 | (1) |
The Tb2O3 thereafter obtained is stable, at least over the range of −log(PO2/atm) = 13.00–4.35.33 In our experiment, oxygen partial pressure is estimated to be 5 × 10−6 atm when the content of the oxygen is 2 ppm and then its logarithm is about 5.3. Anyway, this reaction can occur in the melt and the dark brown Tb4O7 can be successfully transformed into white Tb2O3.
On the other hand, most lanthanide oxides can be readily chloridized by an excessive amount of AlCl3 to generate Ln3+ ions in a LiCl–KCl melt.34,35 Similarly, here Tb2O3 can also react with AlCl3 and the reaction equation can be expressed as follows:
| Tb2O3(s) + Al2Cl6(g,l) → 2TbCl3(l) + Al2O3(s) | (2) |
The Gibbs free energy of this reaction under our experimental condition is calculated to be lower than −388.479 kJ mol−1 at 773 K, implying reaction (2) could proceed forward spontaneously. According to eqn (1) and (2), the following reaction equation can be deduced:
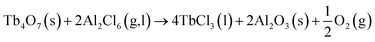 | (3) |
Electrochemical reduction of Tb(III) ions on a Mo electrode
Fig. 1(a) illustrates the typical CV recorded on a Mo electrode in LiCl–KCl–AlCl3 molten salt before (dotted curve) and after (solid curve) the addition of Tb4O7 at a scan rate of 100 mV s−1 and at a temperature of 787 K. The dotted curve exhibits three well-defined couple waves, A/A′, B/B′ and D/D′. The cathodic peak A at about −2.40 V corresponds to the deposition of lithium, its homologous anodic peak A′ represents the dissolution of deposited lithium.5 B/B′ and D/D′ should correspond to the formation and re-oxidation of the Al–Li alloy36 and Al metal, respectively. As can be seen in the solid curve, another couple of peaks, C/C′, appear. The feature of a cathodic signal C rising sharply at around −2.06 V and then decaying slowly suggests a new solid phase, pure Tb metal, may be formed.37Correspondingly, signal C′ should designate the dissolution of Tb metal.
Fig. 1(b) shows the CVs recorded at different scan rates ranging from 50 to 250 mV s−1. It can be seen that the cathodic potential shifts to more negative values and the corresponding anodic peak potential moves toward more positive ones with an increasing scan rate, possibly due to the effect of nucleation over-potential. On the other hand, the linearity of peak currents obtained from the CVs in Fig. 1(b) as a function of the square root of the scan rate in Fig. 1(c) confirms the diffusion-controlled nature of the deposition of electro-active species onto the surface of a molybdenum electrode, revealing that the redox process is reversible. Furthermore, the mid-peak potential is directly proportional to the scan rate, presented in Fig. 1(d). According to these observations, the redox couple can be regarded as reversible.
Compared to CV, SWV can often provide more accurate information not only in the reversible system but also in other extended systems in so far as the peak intensities are linearly related to the square root of the signal frequencies.38,39
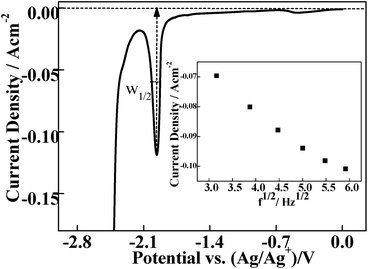
Fig. 2 illustrates a representative SWV of a LiCl–KCl–TbCl3 (0.94 wt%) system at a frequency of 20 Hz and temperature of 787 K, which exhibits an asymmetrical-Gaussian shape peak (peak C) due to the nucleation effect. And yet, the inset of Fig. 2 shows this linear correlation between I and f1/2 in the 5–35 Hz frequency range, implying the system can be considered reversible. It means that eqn (4) can be used to determine the number of exchanged electrons by measuring the half-peak width W1/2 of the asymmetrical peak.40
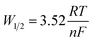 | (4) |
According to the results of Fig. 2, n is calculated to be 2.80 ± 0.20, which is very close to 3. Therefore, peak C should be attributed to the reduction of Tb(III) to Tb(0).
In the chronopotentiometry, two single plateaus can be observed in the LiCl–KCl–TbCl3 (0.90 wt%) melt by applying a current density of −0.096 A cm−2 on a Mo electrode at 787 K (Fig. 3). The plateau at −2.05 V is associated with the reduction of Tb(III) ions. Afterwards, the potential decreases gradually to the limiting value at an electrode potential of −2.42 V, corresponding to the deposition of the lithium metal.
 | ||
| Fig. 3 Typical chronopotentiogram for the reduction of Tb(III) in the melt at the Mo electrode. Current density: 0.096 A cm−2, temperature: 787 K. | ||
Basically, for a semi-infinite linearly diffusion-controlled process, the plot of peak current as a function of the square root inverse of the transition time (inset of Fig. 3) confirms that the Sand equation is appropriate for our system. According to eqn (5), the diffusion coefficient can be determined by using the slope of the curve.
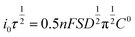 | (5) |
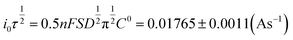 | (6) |
The reversal CP is recorded at several current densities ranging from −0.072 to 0.072 A cm−2, as presented in Fig. 4. It is found that the ratios of the calculated transition times τred/τox are almost close to each other (Table 1), suggesting the formation of insoluble products on the electrode during the cathodic cycle.
 | ||
| Fig. 4 Reversal chronopotentiogram for reduction of Tb(III) in the melt at the Mo electrode. Current density: 0.072 A cm−2, temperature: 787 K, concentration of Tb3+: 0.90 wt%. | ||
| Current density (A cm−2) | τred/τox |
|---|---|
| 0.072 | 0.968 |
| 0.089 | 0.916 |
| 0.107 | 0.997 |
Electrochemical reduction of Tb(III) ions on a solid Al cathode
The phase diagram of the Al–Tb system41 (Fig. 5) shows the presence of five intermetallic compounds, namely, Al2Tb, Al3Tb, AlTb, Al2Tb3 and AlTb2. Thus, in order to identify the formation mechanism of Al–Tb intermetallic compounds in the LiCl–KCl system under our experimental conditions, the electro-reduction of Tb(III) using an Al wire as the working electrode was investigated by CV.Fig. 6 presents the CV of LiCl–KCl–Tb(III) solution at the Mo (curve 2) and Al (curve 1) wires. It was found that the features of the curves obtained by these two electrodes are somewhat different. According to previous results, the cathodic reaction of Tb(III) on the Mo electrode proceeds in a single electrochemical step, as follows:
| Tb(III) + 3e− → Tb(0) | (7) |
 | ||
| Fig. 6 Comparison of CVs obtained on Mo (curve 2) and Al (curve 1) electrodes in the LiCl–KCl–TbCl3 melt, respectively. | ||
When an Al electrode was employed, the underpotential deposition of Tb can take place according to the following reaction:30,42–45
| yTb3+ + xAl + 3(x + y)e− → AlxTby | (8) |
In this case, the equilibrium potential of the Tb3+/TbxAly system can be described by the following equation
 | (9) |
From Fig. 6, a cathodic peak I, which is more positive than peak B, is observed at −1.44 V on the Al electrode. Generally, peak I is due to the under potential deposition of Tb on the Al electrode, which further causes the formation of Al–Tb alloys. The difference between peak I and peak B is related to the activity decrease of Tb in Al–Tb alloys compared to that in pure Tb metal. Similar findings have also been noticed for the reduction of AmCl3 and NdCl3 solutions in a LiCl–KCl eutectic on an the aluminum electrode by Serp et al.46 and Castrillejo et al.47 In our view, this intermetallic phase is related to the formation of an Al-rich intermetallic compound Al3Tb, just because the Al electrode can provide a fertile environment for Al3Tb alloy formation. In addition, the availability of Al3Tb in the Al-rich system is further confirmed by relevant electrolysis experiments.
Electrochemical reduction of Tb(III) ions on an Al-coated Mo electrode
In order to obtain more details about the formation process of Al–Tb intermetallic compounds in this system, the electrochemical behavior of Tb(III) on an Al-coated Mo electrode was systematically studied by different transient techniques. Here, it is noteworthy to mention that no report about the electro-reduction of Tb(III) ions on an Al-coated Mo electrode in LiCl–KCl molten salt has been available in literature.Analysis of cyclic voltammetry
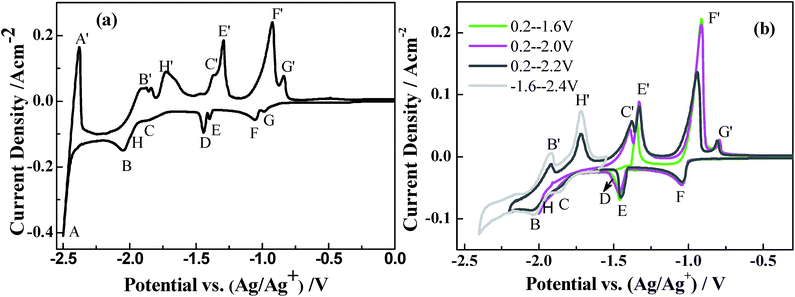
Fig. 7(a) presents the CV curves collected in a LiCl–KCl melt containing AlCl3 (1.5 wt%)–Tb4O7 (1 wt%) at 787 K on the Mo electrode. As discussed above, the redox couple peaks A/A′ are related to the reduction of Li+ and its subsequent re-oxidation, respectively. Signals F/F′ correspond to the deposition and dissolution of the aluminum metal. The peaks G/G′ should be attributed to the formation and dissolution of the Al–Mo alloy.48 Peak B at around −2.05 V and its corresponding anodic peak B′ at around −1.92 V are ascribed to the reduction of Tb(III) to Tb metal and its subsequent re-oxidation, respectively. In addition, four other redox couples, C/C′, D/D′, E/E′ and H/H′, are observed between peaks B/B′ and F/F′. Generally, these peak couples should be associated with the formation of various Al–Tb intermetallic compounds and their subsequent dissolution reactions.In order to facilitate the deposition of Tb(III) ions at the Mo electrode and gain more Al–Tb intermetallic compounds, potentiostatic electrolysis was primarily employed at −1.10 V for 30 s on a Mo electrode. Fig. 7(b) shows the CVs obtained by applying different inversion potentials between −2.4 and 0.2 V in the same system. In this case, a thin layer of Al was previously deposited on the Mo electrode, and therefore an Al-coated Mo electrode was formed. Nevertheless, unlike the deposition of Tb(III) on the Al electrode, multiple Al–Tb intermetallic compounds can be formed by the reduction of Tb(III) on the Al-coated Mo electrode. Interestingly, when an inversion potential of −1.60 V is employed, the cathodic peak D(E) at −1.44 V corresponding to the same anodic peak labeled as E′ can be observed in the CV, which could be due to the dissolutions of two various AlxTby intermetallic compounds caused by intermetallic diffusion. When the inversion potential reaches −2.0 V and −2.2 V, the cathodic peak B can be clearly observed, although peak C becomes inconspicuous. The reason for this phenomenon might be that the reductive potentials of peaks C, H and B are too close to be distinguished. Then their corresponding anodic peaks H′ and C′ are attributed to the dissolution of other new intermetallic compounds containing a higher concentration of Tb. Ref. 11 and 20 have also reported that the closer the reduction potential of an intermetallic compound is to that of the Tb metal, the easier the formation of the intermetallic compounds AlxTby with a higher content of Tb. When the inversion potential attains −2.4 V, the intensity of the well-defined redox peaks C/C′ increases. One reasonable explanation is that more heterogeneous cathodic products can be deposited with an increase of inversion potential. On the other hand, the fast formation of Tb-rich Al–Tb alloys can also account for the change of peak intensity.12,14,18–20
Analysis of square wave voltammetry
SWV, a more sensitive transient method than CV, was employed to further identify the redox couples for the formation of intermetallic compounds in this system. Fig. 8 exhibits the SWV recorded on the Mo electrode at a signal frequency of 20 Hz at 787 K. Four confirmable cathode signals labeled with F, D, C and B are observed. The cathodic peaks D and C at −1.44 V and −1.86 V may be ascribed to the formation of two kinds of Al–Tb intermetallic compounds with different chemical stoichiometries. Here, the absence of peak E, which is clearly observed in the CV scan, suggests that the intermetallic phase associated with peak E might be unstable and easily transformed into another stable intermetallic phase in our experiment. According to the above results, peak F at −1.02 V and peak B at −2.02 V in Fig. 8 could be attributed to the deposition of pure Al metal and the reduction of Tb(III) to Tb(0), respectively. In addition, the small peak A might correspond to the formation of the Al–Li alloy. On the other hand, the shape of signal C is noticeably wide, mainly due to little difference in the reduction potentials between the rich Tb-containing intermetallic compounds AlxTby and the pure Tb metal. | ||
| Fig. 8 Square wave voltammogram of the LiCl–KCl–AlCl3 (1.5 wt%)–Tb4O7 (1 wt%) melts. Working temperature: 787 K, electrode: molybdenum, step potential: 1 mV, frequency: 20 Hz. | ||
Analysis of open circuit voltammetry
OCP is a suitable method for the determination of the formation potential of intermetallic compounds.49,50 In this way, samples of thin layers of Al–Tb intermetallic compounds were prepared by cathodic deposition on the Mo electrode for different short periods of time at potentials of −1.9 V, −2.1 V and −2.4 V, respectively, to ensure the formation of the maximum number of intermetallic compounds. After a short period of cathodic deposition, the transient OCP curve was obtained. With the increase of potential, a series of plateaus can be successively observed and each plateau corresponds to a certain state in which two phases coexist on the Mo electrode surface. In order to confirm the reproducibility of the experiment, the measurement was repeated several times for each deposition. Fig. 9 displays the OCP curve obtained on the Mo electrode in the melt of LiCl–KCl–AlCl3 (1.5 wt%)–Tb4O7 (1 wt%) at 787 K. From Fig. 9, it is noticeable that five well-defined plateaus are present even though a short deposition time was applied. Two distinct potential plateaus B and F observed in the (curve (1)–(3)) are undoubtedly associated with the deposition of pure Tb and Al metal, respectively. On the other hand, the new potential plateau E at around −1.39 V (curve (1)–(3)) and plateau C at −1.76 V are observed in (curve (1) and (2)) after applying a potential of −1.9 V and −2.4 V, respectively, which correspond to the formation of two Al–Tb intermetallic compounds. Moreover, another stripping plateau D at around −1.42 V was not noticeable until a cathodic deposition potential of −1.9 V was applied, as shown in (curve (3)). It is reasonable to attribute this to the formation of another type of Al–Tb intermetallic compound. It should be mentioned that only when the selected potential is a little more negative than that of an alloy, would it be more favorably produced. These five potential plateaus (B–F) are consistent with the previous results obtained in the cyclic and square wave voltammetry. However, a small potential deviation, as shown in Fig. 9, was observed compared with that in Fig. 7 and 8. This might be caused by the incompletely reversible system we used. As a result, under our experimental conditions, three kinds of intermetallic compounds are detected. The reduction extraction of Tb(III) to Tb metal can be realized completely as long as the appropriate conditions are applied.Preparation and characterization of Al–Tb alloys
Based on the results of CV, SWV, and OCP, potentiostatic electrolysis was carried out to prepare Al–Tb alloy samples on Al foils; all the deposition conditions are listed in Table 2. For the deposition time of 5 h at 803 K, a coherent deposited layer that covered the aluminum foil was detected, as shown in Fig. 10(a). The thickness of the deposited layer was measured to be around 150 μm. The XRD pattern shown in Fig. 11(a) confirms that Al and Al2Tb are available on the surface of the aluminum plate. Actually, Li et al. also obtained the intermetallic compound Al2Tb by potentiostatic electrolysis at a potential of −1.8 V at 773 K,51 and Al2Tb was the only intermetallic compound obtained under their experimental conditions. It is reasonable to believe that the Al2Tb phase is quite stable in such systems and is easily formed.| T (K) | CAlCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CTb4O7 (wt%) CTb4O7 (wt%) |
Experimental conditions | The deposits |
|---|---|---|---|
| 803 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
−1.6 V, 5 h | Al2Tb |
| 903 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
−1.6 V, 2 h | Al3Tb |
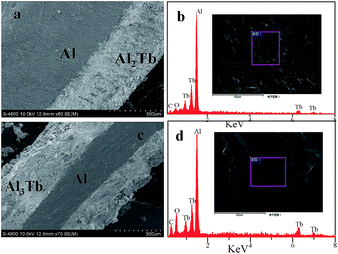 | ||
| Fig. 11 (a–d) Cross-sectional SEM images coupled with the EDS analysis of an Al–Tb film formed by potentiostatic electrolysis at 803 K (a and b) and 903 K (c and d). | ||
Then, the temperature was elevated to 903 K to find out more information about the Al–Tb alloy formation, and a deposition potential of −1.6 V was still applied. It is interesting that the intermetallic compound Al3Tb was observed after 2 h electrolysis. The XRD pattern of this deposition product is shown in Fig. 11(b). One explanation for this phenomenon might be that at a higher temperature the diffusion of the deposited Al and Tb would be promoted, and the deposited Tb would more easily react with the Al deposits in advance, and hence the Al-rich intermetallic compounds AlxTby would be more easily formed. From the XRD analysis, intermetallic compound Al2Tb and Al metal are both observed on the electrode surface at 803 K, whilst only Al3Tb is detected at 903 K. This result suggests that the deposited Al should completely react with the deposited Tb at 903 K. Another explanation for the formation of Al3Tb might be that the Al2Tb phase may be formed initially on the electrode surface and then diffuse into the overall aluminum electrode, and thereafter the transformation of Al2Tb into the more aluminum-rich phase (Al3Tb) would occur. Actually, similar phenomena have also been observed by Liu et al.12,19 and Su et al.12,19
Fig. 11(c and d) present the SEM image coupled with EDS analysis of the deposition samples. As shown in Fig. 11(c), the Al electrode was coated with a uniform thick film of about 500 μm, further confirming the deduction above. Therefore, it can be concluded that, with the alloy growth formed at the Al electrode–electrolyte interface, the inter-diffusion of Al and Tb metal phases can also take place. Once again, the EDS analysis of the surface deposit manifested that intermetallic compounds contain Tb and Al elements. Additionally, the deposits predominantly exist in a blocky shape (see Fig. 11(b and d)).
Conclusions
The electrochemical behaviors of Tb(III) ions on a Mo electrode were investigated in LiCl–KCl–TbCl3 molten salt by using a series of electrochemical techniques (i.e. CV, SWV, and CP). The electro-reduction of Tb(III) on the molybdenum electrode takes place in a single electrochemical process with the exchange of three electrons. The reduction shows a reversible diffusion-controlled mode in the LiCl–KCl eutectic in a scan rate range between 50 and 250 mV s−1. When an Al wire was used as the working electrode, one kind of AlxTby intermetallic compound could be formed. Additionally, aiming at a deeper understanding of the deposition of Al–Tb intermetallic compounds, the Al-coated Mo electrode was employed, and three signals corresponding to the formation of AlxTby intermetallic compounds were observed. Finally, Al–Tb alloy samples were prepared by potentiostatic electrolysis on aluminum foil electrodes. SEM-EDS and XRD analyses demonstrated that a layer of Al2Tb can be formed by potentiostatic electrolysis at a potential of −1.6 V at 803 K, while the Al3Tb phase was obtained by applying a higher temperature at 903 K. The results clearly identified the feasibility of preparing intermetallic compounds Al2Tb and Al3Tb on an Al cathode from molten chlorides and gave some insightful information for the understanding of the extraction of Tb metal from oxide precursors in an LiCl–KCl melt.Acknowledgements
This work was supported by the Major Research Plan “Breeding and Trans-mutation of Nuclear Fuel in Advanced Nuclear Fission Energy System” of the Natural Science Foundation of China (Grants 91226201, 91426302 11275219) and the "Strategic Priority Research program" of the Chinese Academy of Sciences (Grant XDA030104).Notes and references
- M. Gaune-Escard and K. R. Seddon, Molten salts and ionic liquids: never the Twain, Wiley, 2010 Search PubMed.
- K. M. Goff, M. F. Simpson and K. M. Goff, Proceeding of Global, 2009 Search PubMed.
- S. Bourg, C. Hill, C. Caravaca, C. Rhodes, C. Ekberg, R. Taylor, A. Geist, G. Modolo, L. Cassayre, R. Malmbeck, M. Harrison, G. de Angelis, A. Espartero, S. Bouvet and N. Ouvrier, Nucl. Eng. Des., 2011, 241, 3427–3435 CrossRef CAS PubMed.
- L. Cassayre, C. Caravaca, R. Jardin, R. Malmbeck, P. Masset, E. Mendes, J. Serp, P. Soucek and J. P. Glatz, J. Nucl. Mater., 2008, 378, 79–85 CrossRef CAS PubMed.
- S. Ghosh, S. Vandarkuzhali, P. Venkatesh, G. Seenivasan, T. Subramanian, B. Prabhakara Reddy and K. Nagarajan, J. Electroanal. Chem., 2009, 627, 15–27 CrossRef CAS PubMed.
- S. Vandarkuzhali, M. Chandra, S. Ghosh, N. Samanta, S. Nedumaran, B. Prabhakara Reddy and K. Nagarajan, Electrochim. Acta, 2014, 145, 86–98 CrossRef CAS PubMed.
- P. Souček, R. Malmbeck, C. Nourry and J. P. Glatz, Energy Procedia, 2011, 7, 396–404 CrossRef PubMed.
- M. Salanne, C. Simon, P. Turq and P. A. Madden, J. Phys. Chem. B, 2008, 112, 1177–1183 CrossRef CAS PubMed.
- O. Conocar, N. Douyere, J.-P. Glatz, J. Lacquement, R. Malmbeck and J. Serp, Nucl. Sci. Eng., 2006, 153, 253–261 CAS.
- Y. Castrillejo, M. R. Bermejo, E. Barrado, J. Medina and A. M. Martínez, J. Electrochem. Soc., 2006, 153, C713 CrossRef CAS PubMed.
- M. R. Bermejo, F. de la Rosa, E. Barrado and Y. Castrillejo, J. Electroanal. Chem., 2007, 603, 81–95 CrossRef CAS PubMed.
- K. Liu, Y.-L. Liu, L.-Y. Yuan, X.-L. Zhao, Z.-F. Chai and W.-Q. Shi, Electrochim. Acta, 2013, 109, 732–740 CrossRef CAS PubMed.
- Y. Castrillejo, P. Fernández, J. Medina, P. Hernández and E. Barrado, Electrochim. Acta, 2011, 56, 8638–8644 CrossRef CAS PubMed.
- L. Wang, Y.-L. Liu, K. Liu, S.-L. Tang, L.-Y. Yuan, L.-L. Su, Z.-F. Chai and W.-Q. Shi, Electrochim. Acta, 2014, 147, 385–391 CrossRef CAS PubMed.
- P. Taxil, L. Massot, C. Nourry, M. Gibilaro, P. Chamelot and L. Cassayre, J. Fluorine Chem., 2009, 130, 94–101 CrossRef CAS PubMed.
- M. Gibilaro, L. Massot, P. Chamelot and P. Taxil, Electrochim. Acta, 2009, 54, 5300–5306 CrossRef CAS PubMed.
- W. H. Zhang Meng, H. Wei, Z. MiLin, L. YunNa, W. YanLi and M. F. Z. X. X. Yun, Sci. China: Chem., 2014, 57, 1477–1482 CrossRef.
- K. Liu, Y.-L. Liu, L.-Y. Yuan, X.-L. Zhao, H. He, G.-A. Ye, Z.-F. Chai and W.-Q. Shi, Electrochim. Acta, 2014, 116, 434–441 CrossRef CAS PubMed.
- L.-L. Su, K. Liu, Y.-L. Liu, L. Wang, L.-Y. Yuan, L. Wang, Z.-J. Li, X.-L. Zhao, Z.-F. Chai and W.-Q. Shi, Electrochim. Acta, 2014, 147, 87–95 CrossRef CAS PubMed.
- Y.-L. Liu, Y.-D. Yan, W. Han, M.-L. Zhang, L.-Y. Yuan, K. Liu, G.-A. Ye, H. He, Z.-F. Chai and W.-Q. Shi, RSC Adv., 2013, 3, 23539 RSC.
- G. Qiu, D. Wang, X. Jin and G. Z. Chen, Electrochim. Acta, 2006, 51, 5785–5793 CrossRef CAS PubMed.
- G. Qiu, D. Wang, M. Ma, X. Jin and G. Z. Chen, J. Electroanal. Chem., 2006, 589, 139–147 CrossRef CAS PubMed.
- Q. Rong and H. J. Schaller, J. Alloys Compd., 2004, 365, 188–196 CrossRef CAS.
- Y. Castrillejo, P. Hernández, R. Fernández and E. Barrado, Electrochim. Acta, 2014, 147, 743–751 CrossRef CAS PubMed.
- H. Konishi, K. Mizuma, H. Ono, E. Takeuchi, T. Nohira and T. Oishi, 2012.
- W. Han, Q. Sheng, M. Zhang, M. Li, T. Sun, Y. Liu, K. Ye, Y. Yan and Y. Wang, Metall. Mater. Trans. B, 2014, 45, 929–935 CrossRef CAS.
- M. R. Bermejo, J. Gómez, A. M. Martínez, E. Barrado and Y. Castrillejo, Electrochim. Acta, 2008, 53, 5106–5112 CrossRef CAS PubMed.
- B. Y. Kim, D. H. Lee, J.-Y. Lee and J.-I. Yun, Electrochem. Commun., 2010, 12, 1005–1008 CrossRef CAS PubMed.
- S. Rayaprolu and D. Chidambaram, ECS Trans., 2014, 58, 51–66 CrossRef PubMed.
- W. Xiao and D. Wang, Chem. Soc. Rev., 2014, 43, 3215–3228 RSC.
- D. Wang, G. Qiu, X. Jin, X. Hu and G. Z. Chen, Angew. Chem., Int. Ed. Engl., 2006, 45, 2384–2388 CrossRef CAS PubMed.
- Z. C. Kang and L. Eyring, J. Alloys Compd., 1997, 249, 206–212 CrossRef CAS.
- K. Kitayama, M. Kobayashi, H. Takano, N. Nambu and H. Hirasawa, J. Solid State Chem., 2003, 176, 151–158 CrossRef CAS.
- K. B. a. F. D. G. Steldl, J. Phys. Chem. C, 1983, 84, 5011–5016 Search PubMed.
- G. N. P. a. G. H. KUCERA, Inorg. Chem., 1979, 18, 385–389 CrossRef.
- Y. Fung, D. Inman and S. White, J. Appl. Electrochem., 1982, 12, 669–680 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods, Wiley, New York, 2nd edn, 2001 Search PubMed.
- B. L. a. P. T. P. Chamelot, Electrochim. Acta, 1998, 43, 607–616 CrossRef.
- P. Chamelot, P. Taxil and B. Lafage, Electrochim. Acta, 1994, 39, 2571–2575 CrossRef CAS.
- C. Hamel, P. Chamelot and P. Taxil, Electrochim. Acta, 2004, 49, 4467–4476 CrossRef CAS PubMed.
- L. Jin, Y.-B. Kang, P. Chartrand and C. D. Fuerst, Calphad, 2010, 34, 456–466 CrossRef CAS PubMed.
- H. Yin, W. Xiao, X. Mao, H. Zhu and D. Wang, J. Mater. Chem. A, 2015, 3, 1427–1430 CAS.
- H. Yin, D. Tang, X. Mao, W. Xiao and D. Wang, J. Mater. Chem. A, 2015, 3, 15184–15189 CAS.
- W. Xiao, X. Wang, H. Yin, H. Zhu, X. Mao and D. Wang, RSC Adv., 2012, 2, 7588 RSC.
- W. Xiao, X. Jin and G. Z. Chen, J. Mater. Chem. A, 2013, 1, 10243 CAS.
- J. Serp, M. Allibert, A. L. Terrier, R. Malmbeck, M. Ougier, J. Rebizant and J.-P. Glatz, J. Electrochem. Soc., 2005, 152, C167 CrossRef CAS PubMed.
- Y. Castrillejo, M. R. Bermejo, A. I. Barrado, R. Pardo, E. Barrado and A. M. Martínez, Electrochim. Acta, 2005, 50, 2047–2057 CrossRef CAS PubMed.
- Y. Yan, X. Li, M. Zhang, H. Tang, W. Han, Y. Xue and Z. Zhang, Energy Procedia, 2013, 39, 408–414 CrossRef CAS PubMed.
- Y. Castrillejo, P. Fernández, J. Medina, M. Vega and E. Barrado, Electroanalysis, 2011, 23, 222–236 CrossRef CAS PubMed.
- L. Massot, P. Chamelot and P. Taxil, Electrochim. Acta, 2005, 50, 5510–5517 CrossRef CAS PubMed.
- M. Li, Q. Gu, W. Han, Y. Yan, M. Zhang, Y. Sun and W. Shi, Electrochim. Acta, 2015, 167, 139–146 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |