Temperature-induced work function changes in Mn1.56Co0.96Ni0.48O4 thin films
Abstract
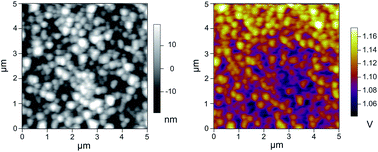
The variations of work functions in Mn1.56Co0.96Ni0.48O4 (MCN) thin films are investigated in the temperature range from 30 to 80 °C. The high resolution images of the contact potential difference (CPD) of MCN thin films were obtained through Kelvin probe force microscopy (KPFM) and the correlations between the work functions and temperatures were demonstrated through the imaginary part of the dielectric functions. The complex dielectric spectra are temperature dependent while their intensities have the inverse trend according to the work functions. The phenomenon can be interpreted by different chemical states that relate to the Mn3+ state.

 Please wait while we load your content...
Please wait while we load your content...