Development and cell imaging applications of a novel fluorescent probe for Cu2+†
Abstract
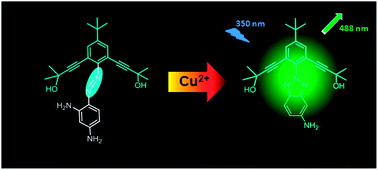
A reactivity-based fluorescent probe 1 for Cu2+ was synthesized. The structure of this probe was characterized by infrared spectroscopy (IR), mass spectrometry (MS), X-ray crystallography, and 1H NMR and 13C NMR spectroscopy. Its photophysical as well as binding properties towards various metal ions were studied. Probe 1 showed highly sensitive and selective “Off-On” fluorescence changes with Cu2+ among various metal ions when excited at 350 nm. These selective changes were attributed to an oxidative cyclization reaction transforming nonemissive azoanilines into highly fluorescent benzotriazoles. Furthermore, fluorescence imaging experiments of Cu2+ ions in living cells demonstrated its value of practical applications in biological systems.

 Please wait while we load your content...
Please wait while we load your content...