Single and bicomponent anionic dyes adsorption equilibrium studies on magnolia-leaf-based porous carbons†
Huijing Yua,
Tingting Wanga,
Wei Dai*a,
Xianxing Lia,
Xin Hua and
Na Ma*b
aCollege of Chemistry and Life Science, Zhejiang Normal University, Jinhua 321004, Zhejiang Province, People's Republic of China. E-mail: daiwei@zjun.edu.cn; Fax: +86-579-82282531; Tel: +86-579-82282269
bCollege of Geography and Environmental Sciences, Zhejiang Normal University, Zhejiang Province, Jinhua 321004, People's Republic of China. E-mail: mana@zjun.cn; Fax: +86-579-82283595; Tel: +86-579-82283595
First published on 13th July 2015
Abstract
A new type of porous carbon is prepared by cost-effective pyrolysis carbonization and subsequent alkali activation of an easily available biomass, magnolia leaf (ML). The as-prepared ML porous carbons (MPCs) show high specific surface areas and suitable pore size distributions. Surface characterization of ML and MPC-1 were investigated by N2 adsorption, FT-IR, SEM and TEM. Two anionic azo dyes were used, namely, orange II (OII) and methyl orange (MO), to simulate the textile effluent. Batch experiments of OII and MO in a single dye system (SDS) and a binary dye system (BDS) onto MPC-1 were investigated as a function of pH, contact time and species concentration. The adsorption process followed the Langmuir isotherm model with high coefficients of correlation (R2 > 0.999). The pseudo-second order kinetic model fitted well to the experimental results. This study indicates that MPCs demonstrated superior OII and MO adsorption capabilities and could be employed as a low cost alternative to commercially available porous carbon for the removal of dyes from wastewater.
1. Introduction
Dyes discharged together with industrial textile wastewaters are main organic pollutants because they are highly visible and undesirable even at low concentrations in water.1 Discharging of dye-containing effluents into the hydrosphere is prohibited not only because of their color, which reduces the sunlight penetration into the water, but also because of the toxic, carcinogenic and mutagenic nature of their breakdown products.2,3 Among different types of dyes, OII and MO are well-known typical acidic/anionic and azo dyes, and have been widely used in textile, printing, paper, food and pharmaceutical industries as well as research laboratories.3 The structures of OII and MO are similar and are shown in Scheme 1. As demonstrated in Scheme 1, due to one more benzene ring in the structure, the molecular weight and size of OII are larger than MO as the typical representative azo dyes. Acute exposure to OII and MO can cause increased heart rate, vomiting, shock, cyanosis, jaundice, quadriplegia, and tissue necrosis in humans.4 Thus, simultaneous removal of OII and MO from wastewater and/or process effluent is very important due to their potential toxicity to humans and the environment. OII and MO, for the reasons stated above, were selected in this study as the textile dye effluent models.Various methods, such as chemical precipitation,5,6 ion exchange,7,8 membrane filtration,9,10 physical adsorption,11,12 chemical oxidation/reduction,13,14 and bioremoval,15,16 have been developed for the removal of dyes from wastewater. Among these, the adsorption technique is considered to be a competitive method for the treatment of dyestuff wastewater due to its easy handling, high efficiency, and economic feasibility.17 The adsorbents with high capacity and high rate play a critical role in the removal of dye molecules by adsorption. Porous carbon material (PCM) is a commercial adsorbent for eliminating pollutants from wastewater and air. However, the higher production cost and difficulty in PCM regeneration limit its widespread use.18,19 Currently, there are many studies on the development of low-cost adsorbents such as the use of waste materials for this purpose.20 PCM can be generally acquired by simple carbonization and activation treatment of cheap and easily available natural biomass wastes or carbonaceous minerals using different porogens.20,21 Hence, the preparation of PCM from natural feedstock has received extensive interest. All types of raw materials, including sugar cane bagasse,22 tea leaves,23 pulps, peels or seeds of fruits,24–26 sunflower seed shell,27 porous starch,28 coal tar pitch,29,30 have been widely employed as precursors to obtain porous carbons as adsorbents for the removal of specific pollutants from an aqueous phase.
Magnolia, a type of deciduous tree, is widely grown in most regions of China because of its robust cold and drought tolerance. As a rather low, rounded, thickly branched, and coarse-textured tree, the height of the magnolia tree can reach 30 feet (9.1 m). The magnolia leaves (MLs) are ovate, bright green, 15 cm long and 8 cm wide, which endow MLs with good antiviral and antibacterial efficacies.31 Nevertheless, in most cases, MLs are discarded and rotted away into soil without effective utilization. Given its porous texture and the fact that it contains various organic compounds, ML is an optimal precursor for the preparation of porous carbon material (magnolia-leaf-based porous carbons, MPCs) and it can also be expected to adsorb dyes.
In this study, we synthesized a novel three-dimensional magnolia-leaf-based porous carbon by high temperature carbonization and alkali activation. Two organic dyes (OII and MO) with similar structures were used as model pollutants to investigate the adsorption characteristics of the as-prepared adsorbent in SDS and BDS systems. The remarkably enhanced specific surface areas, versatile pore texture with the coexistence of micro-pores and meso-pores, increased hydrophilicity and moderate graphitization make the as-prepared MCPs high performance adsorbers of OII and MO from an aqueous solution. The influence of several operating parameters, such as dye concentration, contact time, adsorbent dosage, pH and temperature, was investigated. Equilibrium isotherms and kinetics modelling were used to investigate the possible mechanism of the adsorption process.
2. Experimental section
2.1. Materials
Potassium hydroxide (KOH), hydrochloric acid (HCl) and sodium hydroxide (NaOH) used in this study were purchased from Sinopharm Chemical Reagent Co., Ltd, China. They were of analytical grade and were used as received without further purification. An aqueous stock solution of OII and MO was prepared by dissolving OII (C16H11N2NaO4S, MW: 350.32, Sigma-Aldrich) and MO (C14H14N3NaO3S, MW: 327.34, Sigma-Aldrich) in deionized water. A type of commercial active carbon, namely, coconut shell-based activated carbon (CS-AC), was obtained from Chemical Reagent Co., Ltd, China. The BET surface area and total volume of CS-AC is 1202 m2 g−1 and 1.07 cm3 g−1, respectively. Deionized water was used to prepare the desired concentrations of dye wastewater model.2.2. Preparation of MPCs
MPCs were prepared by a similar KOH activation procedure that was previously proposed by our group.2,19 In a typical preparation, 2 g of precursor (MLs) with a particle size of 150 μm was mixed with 8 g of KOH powder. Hence, the mass ratio of KOH and MLs precursor was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the resulting mixture was placed into a horizontal pipe reactor (o.d., 50 mm) and activated as follows. (i) Nitrogen gas was allowed to flow through the reactor at a rate of 40 mL min−1 and maintained at this flow rate throughout the activation process. (ii) The temperature of the reactor was increased to the activation temperature (1073 K) at a heating rate of 1 K min−1. (iii) The reactor was held at the activation temperature for 40 min. (iv) Finally, it was cooled to room temperature. The samples thus obtained were washed with distilled water until the filtrate appeared neutral. The final samples (MPC-1) were obtained by heating these samples at 423 K under vacuum for 24 h.
1, and the resulting mixture was placed into a horizontal pipe reactor (o.d., 50 mm) and activated as follows. (i) Nitrogen gas was allowed to flow through the reactor at a rate of 40 mL min−1 and maintained at this flow rate throughout the activation process. (ii) The temperature of the reactor was increased to the activation temperature (1073 K) at a heating rate of 1 K min−1. (iii) The reactor was held at the activation temperature for 40 min. (iv) Finally, it was cooled to room temperature. The samples thus obtained were washed with distilled water until the filtrate appeared neutral. The final samples (MPC-1) were obtained by heating these samples at 423 K under vacuum for 24 h.
2.3. Porous carbon characterization
Specific surface areas and pore volumes were determined by N2 adsorption. An automated adsorption apparatus (Micromeritics, ASAP 2020) was used for the measurements. N2 adsorption was carried out at liquid N2 temperature (77 K). The specific surface areas were calculated using the BET equation by assuming a section area of nitrogen molecule to be 0.162 nm2. The t-plot method was applied to calculate the micro-pore volumes and surface areas. The total pore volume was estimated to be the liquid N2 volume at a relative pressure of 0.99. The pore size distribution was calculated by density functional theory (DFT) calculations. The morphologies of the prepared MPCs and raw material (MLs) were examined by scanning electron microscopy (SEM) (300 K, Pixel CMOS). FT-IR spectra for the samples were recorded on a Nexys 670 FT-IR spectrometer (Nicolet Instrument Co., USA). The transmission spectra of the samples were recorded using KBr wafers containing 0.5 wt% of the samples. These wafers were dried overnight at 393 K before the spectra were recorded. The spectra were obtained using 64 scans with a resolution of 4 cm−1.2.4. Batch equilibrium studies
The equilibrium isotherms of OII and MO adsorption on MPC-1 were determined by performing adsorption tests in 100 mL Erlenmeyer flasks, where 50 mL of OII and MO solutions with different initial concentrations (100–1000 mg L−1) were placed in each flask. To determine the adsorption capacities at various pH values, the pH of the dye solutions was adjusted with aqueous solutions of 0.1 M HCl or 0.1 M NaOH. 0.02 g of each of the prepared MPC-1 with particle size of 110 μm were added to each flask and kept in a shaker at 150 rpm at room temperature (298 K) for 3 h to reach equilibrium. Then, the samples were filtered and the residual concentrations of OII and MO in the filtrate were analyzed using a UV-Visible spectrophotometer (Thermo Fisher Evolution 300 PC) at maximum wavelengths of 485 and 464 nm, respectively. It was found that the calibration curves were reproducible and linear over the concentration range used in this study. The adsorbed amount of OII and MO at equilibrium, qe (mg g−1), was calculated by the following expression:
 | (1) |
The batch experiments of binary dye solution were performed with a similar procedure. In a binary system with components A (OII) and B (MO), the measurements were carried out at their maximum absorbance wavelength λmax1 and λmax2, giving absorbances of A1 and A2, respectively. Concentrations of dye solution were then estimated quantitatively using the linear regression equations obtained by plotting a calibration curve for each dye over a wide range of concentrations. In a binary dye solution, the concentrations of OII and MO were calculated according to the literature.32 In a binary system with components A and B, dye concentrations were calculated by the following equations:32
 | (2) |
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w).
1 (w/w).
3. Results and discussion
3.1. Material characterization
The nitrogen adsorption isotherm graph of MPC-1 at 77 K is shown in Fig. 1. The isotherm displays type-I shape according to the IUPAC classification.17,19 The pore-size distribution calculated by density functional theory (DFT) proved that the pore size of MPC-1 mainly focused on 2 nm. The specific BET surface area and pore volume of MPC-1 sample were 2834 m2 g−1 and 1.58 cm3 g−1, respectively. The huge surface area of MPC-1 could provide huge capacity for the adsorption of dye molecules inside the pore structure. The SEM micrographs of ML and MPC-1 are presented in Fig. 2 to demonstrate their surface shape. Based on the analysis of the images obtained by SEM before and after the KOH chemical activation process, two distinct types of shapes were observed. The surface of ML (Fig. 2(A)) was fairly smooth with few cracks or voids, while the SEM micrograph (Fig. 2(B)) of MPC-1 particles showed cavities, pores and more rough surfaces due to the carbonization and KOH activation process. The surface area of MPC-1 was enhanced by the presence of more porosity, which could hold more dye molecules from solution during adsorption. This means that KOH activation has an influence on pore development during the preparation process. This phenomenon of the activation process is found similar to previous studies.2,19 FT-IR transmission spectra (Fig. 3) were obtained to characterize the surface groups of ML and MPC-1 samples. As shown in Fig. 3, the FTIR spectroscopic analyses of MPC-1 and ML indicated broad bands at 3440 cm−1, representing bonded –OH groups. The band at 2930 cm−1 indicates the presence of an aliphatic –CH stretching. The band at 2358 cm−1 is the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching vibrations in alkyne groups. The band at 1728 cm−1 denotes the existence of carbonyl/carboxyl groups. The band at 1610 cm−1 indicates the presence of an aromatic C
C stretching vibrations in alkyne groups. The band at 1728 cm−1 denotes the existence of carbonyl/carboxyl groups. The band at 1610 cm−1 indicates the presence of an aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ring stretching. The bands observed at 1388 cm−1 could be assigned to the symmetric bending of C–H. The band at 1068 cm−1 indicates the C–O stretching vibrations in alcohols, phenols or ether or ester groups. The peak observed at 400–700 cm−1 could be attributed to C–C stretching. After KOH activation process, many groups of ML are destroyed and they possess aromatic structure and oxygen groups. Thus, the FTIR analysis indicates that the MPC-1 is characterized by functional groups such as O–H, COOH, CO, C–C, C
C ring stretching. The bands observed at 1388 cm−1 could be assigned to the symmetric bending of C–H. The band at 1068 cm−1 indicates the C–O stretching vibrations in alcohols, phenols or ether or ester groups. The peak observed at 400–700 cm−1 could be attributed to C–C stretching. After KOH activation process, many groups of ML are destroyed and they possess aromatic structure and oxygen groups. Thus, the FTIR analysis indicates that the MPC-1 is characterized by functional groups such as O–H, COOH, CO, C–C, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, which could be potential adsorption sites for interaction with the anionic dyes. This finding is similar to that found in the literature.33,34
C, which could be potential adsorption sites for interaction with the anionic dyes. This finding is similar to that found in the literature.33,34
3.2. Effect of adsorbent dosage and pH
The dependence of OII and MO adsorption on the concentration of model wastewater in SDS and BDS systems was investigated by varying the quantity of the MPC-1 adsorbent from 0.005 to 0.05 g in 50 mL of 1000 mg L−1 solution of the dye while keeping other parameters (contact time = 3 h, agitation speed = 150 rpm, particle size = 110 μm, temperature = 298 K) constant. As shown in Fig. 4, the adsorption capacities of OII and MO in the first stage increase rapidly with the increase in the adsorbent dose, and then the adsorption capacities are not changed significantly with the further increase in the adsorbent dose. It can be seen that the adsorption capacities of the dyes reach saturation with the adsorbent dose more than 0.4 g L−1. Thus, 0.4 g L−1 was chosen as the optimum dose and used in further experiments. The two-stage-dependent adsorption behavior was also in agreement with the literature.19,35 This observation is usually attributed to the increase in the adsorbent pore surface areas and availability of more adsorption sites with increasing mass of adsorbent.The effect of the solution pH on the adsorption of dyes on MPC-1 in SDS and BDS systems is shown in Fig. 5. For both OII and MO, the adsorption capacities at low pH were larger than those at high pH in both SDS and BDS systems. In addition, the effects of solution pH for OII and MO adsorption were different. When the solution pH was varied from 2 to 10, the OII and MO uptake capacities in SDS decreased from 1920 and 1419 mg g−1 to 1488 and 869 mg g−1 (decrease of 26% and 39%, respectively). However, their capacities in BDS decreased from 1094 and 616 mg g−1 to 951 and 447 mg g−1 (decrease of 13% and 27%, respectively). This discrepancy suggests the presence of the competitive adsorption for OII and MO adsorption onto MPC-1 in BDS.
The mechanism of the adsorption of dyes onto porous carbons is controlled by various factors such as physical and/or chemical properties of porous carbon, molecular structure of dyes, hydrophobic bonds, electrostatic force, mass transfer process, van der Waals force and hydrogen bond formation.17,19,35 As shown in Scheme 1, OII and MO are ideally planar molecules and therefore can easily adsorb on MPC-1 by π–π stacking interaction between the aromatic backbone of the dye and the hexagonal skeleton of MPC-1. Thus, we think that the larger uptake capacity of OII than MO comes from the fact that OII structure has a stronger π–π stacking interaction with MPC-1 due to its expanded aromatic ring. Such a finding is similar to that made in previous studies.17,19 Usually, there are oxygen-containing groups at the surface of the porous carbon (COOH, C–OH, etc.). Thus, the surface charge of the porous carbon is dependent on the solution pH. At high solution pH, the porous carbon becomes negatively charged, and the anionic dyes investigated in this study experience an electrostatic repulsion force upon adsorbing onto the surface of the porous carbon. However, at an appropriate low solution pH, the porous carbon is electrically neutral and thus favors the dye adsorption, as observed in Fig. 5, with a higher adsorption capacity for OII and MO in an acidic solution. Therefore, we think that both the electrostatic force and π–π stacking interactions contribute to the dye adsorption.
3.3. Adsorption isotherms
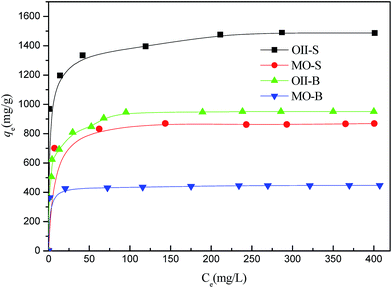
The experimental data at equilibrium between the amount of dye adsorbed (in SDS and BDS) (qe) on the adsorbent (MCP-1) and the concentration of dye solution (Ce) at a constant temperature and pH were used to describe the optimum isotherm model. The linear forms of Langmuir,36 Freundlich,37 Temkin38 and D–R39 isotherm model equations were used to describe the equilibrium data. Applicability of these equations was judged by comparing the correlation coefficients (R2). In this study, adsorption isotherm experiments were carried out at initial dye concentrations of 100–1000 mg L−1. Isotherm parameters, evaluated from the linear plots of eqn (4–7), are illustrated in Table S.I.1.† The values of qm and KL are presented in Table S.I.2.† The qm values for the Langmuir I isotherm in SDS system are 1488 (OII) and 869 (MO) mg g−1, whereas in BDS, they are 951 (OII) and 447 (MO) mg g−1. The adsorption coefficients (KL, related to the apparent energy of sorption) were found to be 0.25 (OII) and 0.50 (MO) for SDS and 0.21 (OII) and 0.41 (MO) L g−1 for BDS. The R2 (correlation coefficient) value of >0.999 indicates that the Langmuir isotherm is efficient for explaining the sorption of OII and MO on MPC-1 in two systems. The uptake capacities of the anionic dyes onto the MPC-1 were found to be lower in BDS than in SDS due to their competition adsorption.To investigate the possible multilayer adsorption and non-linear energy distribution of the adsorption sites, the Freundlich isotherm was studied. The intercept value (Kf) and the slope n (Table S.I.2†) were obtained from the linear plots of Freundlich isotherm (not shown) at room temperature (298 K). The values of R2 for Freundlich plots were 0.9855 (OII) and 0.8789 (MO) for SDS and 0.9417 (OII) and 0.9178 (MO) for BDS. The values of 1/n were 0.07 (OII) and 0.05 (MO) for SDS and 0.12 (OII) and 0.03 (MO) for BDS. The value of 1/n (indicative of favorability) from Freundlich isotherm model, ranging from 0 to 1, is a measure of adsorption intensity or surface heterogeneity, which shows more heterogeneity as its value gets closer to zero. A value for 1/n below 1 indicates a normal Langmuir adsorption isotherm, while a value of 1/n above 1 is indicative of cooperative adsorption.
Temkin adsorption was chosen to fit the equilibrium adsorption data. The parameters KT and bT of the Temkin equation have been calculated for OII and MO adsorption in both systems (Table S.I.2†). The Temkin adsorption potential (KT) values were found to be 6.6 × 104 (OII) and 5.7 × 106 (MO) L g−1 for SDS and 582 (OII) and 4.4 × 1011 (MO) L g−1 for BDS. The Temkin constant (bT) values, related to the heat of sorption, were found to be 28 (OII) and 60 (MO) kJ mol−1 for SDS and 30 (OII) and 180 (MO) kJ mol−1 for BDS. However, the typical range of bonding energy for an ion-exchange mechanism is 8–16 kJ mol−1.19 Because the range of bonding energy associated with the adsorbent under study was found to be substantially low, the interaction between dyes and MPC-1 in both systems appears not to have involved an ion-exchange mechanism, rather a physisorption mechanism.
The Dubinin–Radushkevich (D–R) model is often used to estimate the characteristic porosity and the apparent free energy of adsorption. The isotherm parameters from the linear plots of the isotherm (not shown) are given in Table S.I.2.† Although the adsorbent showed relatively lower R2 values (0.7117 (OII) and 0.9609 (MO) for SDS and 0.5366 (OII) and 0.9232 (MO) for BDS) compared to the preceding models (i.e., Langmuir, Freundlich and Temkin isotherms), the results are significant enough for deriving information regarding the adsorption. The values of sorption affinity (qs) of dyes for MCP-1 as per D–R model are 1393 (SDS) and 827 mg g−1 (BDS). The calculated mean energy of adsorption, E, from the D–R isotherm gives information about the chemical or physical properties of the sorption. The calculated mean energy value of adsorption of dyes by MCP-1 is low (5 kJ mol−1), and this implies that the type of adsorption appears to be due to physical processes because chemisorption processes have adsorption energies greater than 20 kJ mol−1.19
The equations of a competitive Langmuir model28 are as follows:
 | (4) |
 | (5) |
Adsorption isotherms of OII and MO on MPC-1 in SDS and BDS are shown in Fig. 6. The adsorption capacities of OII and MO onto MPC-1 were reduced from 1488 mg g−1 (SDS) to 951 mg g−1 (BDS) and from 869 mg g−1 (SDS) to 447 mg g−1 (BDS) at pH 7, respectively, because of the high contention on active sites between two types of dye molecules in the binary system. MO has a lower molecular weight than OII, and the adsorption capacity of OII is higher than that of MO at pH 7. The experimental data in the binary systems were applied to the competitive Langmuir equations (Table S.I.3†). The correlation coefficients in the binary system were lower than in single dye system. We can say that the adsorbent surface is homogeneous and there is no interaction between the adsorbed molecules. These systems are applied to real systems due to the effect of surface heterogeneity and interaction between azo dye molecules. The MO and OII adsorption performances of CS-AC and ML samples were investigated by the batch test (the experimental conditions are same as that found in Section 2.4). As a result, the OII uptake capacities of CS-AC and ML are 322 and 128 mg g−1 in SDS and 286 and 79 mg g−1 in BDS. The MO uptake capacities of CS-AC and ML are 315 and 115 mg g−1 in SDS and 280 and 72 mg g−1 in BDS. The adsorption capacities of OII and MO on CS-AC and ML in this study are displayed in Table S.I.4.† It is clear that the adsorption capacity of MPC-1 is superior to that of coconut shell-based commercial activated carbon (CS-AC), ML and some other previously reported adsorbents.4,19,21,40–42
3.4. Adsorption kinetics
The kinetics of adsorption is important because it controls the efficiency of the process and the time to reach equilibrium. It also describes the rate of adsorbate uptake on nanoporous carbon. To identify the potential rate-controlling steps involved in the process of adsorption, three kinetic models were studied and used to fit the experimental data from the adsorption of dyes onto nanoporous carbon. These models are the pseudo-first-order,43 pseudo-second-order44 and intra-particle diffusion models.45 These models can be expressed as follows:| Pseudo-first order model: −ln(qe − qt) = ln(qe) − K1t | (6) |
 | (7) |
| Intra-particle diffusion model: qt = K3t1/2 | (8) |
To quantitatively compare the applicability of different kinetic models for fitting the data, a normalized standard deviation, Δq (%), was calculated as follows:
 | (9) |
The effect of contact time on adsorption capacities of MPC-1 for OII and MO from single and binary dye solutions is shown in Fig. 7. These figures show that the adsorption capacities for OII and MO increase with the increase of contact time, and the adsorption reaches equilibrium within about 1 h. The saturation capacities of OII and MO onto MPC-1 in the single dye solution are 1488 and 869 mg g−1 at room temperature (298 K), contact time of 3 h, initial concentration of 1000 mg L−1, pH value of 7 and adsorbent dose of 0.4 g L−1. For single and binary dye adsorption systems, the adsorption capacities of OII and MO were reduced from 1488 mg g−1 (single system) to 869 mg g−1 (binary system) and from 869 mg g−1 (single system) to 447 mg g−1 (binary system), respectively, at pH 7 because of the high contention for active sites between two types of dye molecules in the binary system. The capacity is constant when all the parameters are fixed, and with the evolution of time, the uptake or adsorbed amount may change. The fast adsorption at the initial stage may be due to the availability of the uncovered surface area and the high amount of active sites on the adsorbent.
 | ||
| Fig. 7 Effect of contact time on the adsorption capacities of OII and MO on MPC-1 in SDS and BDS systems. | ||
The experimental kinetic data of OII and MO, calculated from eqn (6)–(7), were correlated by three kinetic models: pseudo-first order, pseudo-second order and intra-particle diffusion models. The calculated constants of the three kinetic equations along with R2 values at different temperatures are presented in Table S.I.5.† As seen in Table S.I.5,† there is a large difference between the experimental and calculated adsorption capacity values when the pseudo-first order model was applied. However, high R2 values (>0.999) are obtained with the linear plot of t/qt versus t, suggesting that the pseudo-second order model of adsorption kinetics should be applied. In addition, the pseudo-second order kinetic model is in good agreement with the experimental and calculated adsorption capacity values, Δq (%). If the intra-particle diffusion is the mechanism of the adsorption process, then the plot of qt versus t1/2 will be linear, and if the plot passes through the origin, then the rate limiting process is due only to the intra-particle diffusion. Otherwise, some other mechanism along with intra-particle diffusion is also involved.33 However, as shown in Fig. 8, the plots are not linear over the whole time range and, instead, can be separated into multi-linear curves, illustrating that multiple stages were involved in the adsorption process. The first straight portion was attributed to the macro-pore diffusion (phase I) and the second linear portion was attributed to micro-pore diffusion (phase II). The results indicate that the adsorption of OII and MO dyes onto MPC-1 involve more than one process, and the intra-particle transport is not the rate-limiting step. Such a finding is similar to that made in previous studies.3,19,35
 | ||
| Fig. 8 Weber–Morris intra-particle diffusion plots for the adsorption of OII and MO on MPC-1 in SDS and BDS. | ||
3.5. Desorption process
Generally, there are two techniques for the desorption of the adsorbent: the thermal treatment and the solvent elution.46,47 Based on the solvent elution procedures mentioned in the literature,46–48 the reservation of the dyes on the MPC-1 is about 80% after washing with organic solvent, acid or alkali media. The results demonstrate that MPC-1 has a relatively good adsorption ability for anionic dyes (OII and MO). In addition, although nearly 90% of the MPC-1 saturated by the dyes can be regenerated after thermal treatment under a stream of nitrogen at 973 K, we did not attempt to cyclically utilize the porous carbon due to its low cost in preparation.4. Conclusion
The present study shows that the MPC-1 obtained from biomass waste is an effective adsorbent for the removal of OII and MO from single and binary dye aqueous solutions. The adsorption ability of the batch adsorption tests demonstrate that the adsorption is affected by various conditions such as contact time, solution pH and initial dye concentration. The equilibrium data for the removal strongly follow the Langmuir monolayer adsorption model with high adsorption capacity in a short amount of time. The kinetics studies showed the applicability of the pseudo-second-order model. Weber–Morris plot verified the adsorption mechanism was due to a multi-linearity correlation. The π–π stacking interaction between the surface of porous carbon and dyes could be one of the important reasons for the high adsorptive performance of MPC-1. Therefore, we conclude that MPC materials can be used as highly efficient adsorbents and can be reused for the removal of anionic azo-dyes from wastewater.Acknowledgements
This study was supported by the Public Projects of Zhejiang Province of China (No. 2015C31083) and Zhejiang Qianjiang Talent Project (No. QJD1302014).Notes and references
- J. Li, D. Ng, P. Song, C. Kong, Y. Song and P. Yang, Biomass Bioenergy, 2015, 75, 189 CrossRef CAS PubMed.
- W. Dai, H. Yu, N. Ma and X. Yan, Korean J. Chem. Eng., 2015, 32, 335 CrossRef CAS.
- L. Shao, Y. Yao, S. Quan, H. Wei, R. Wang and Z. Guo, Mater. Lett., 2014, 114, 111 CrossRef CAS PubMed.
- C. Hsiu-Mei, C. Ting-Chien, P. San-De and C. Hung-Lung, J. Hazard. Mater., 2009, 161, 1384 CrossRef PubMed.
- J. S. An, Y. J. Back, K. C. Kim, R. Cha, T. Y. Jeong and H. K. Chung, Environ. Technol., 2014, 35, 1668 CrossRef CAS PubMed.
- C. R. Li, Z. Y. Zhuang, F. Huang, Z. C. Wu, Y. P. Hong and Z. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 9719 CAS.
- X. Du, H. Zhang, X. Hao, G. Guan and A. Abudula, ACS Appl. Mater. Interfaces, 2014, 6, 9543 CAS.
- L. Fu, C. Shuang, F. Liu, A. Li, Y. Li, Y. Zhou and H. Song, J. Hazard. Mater., 2014, 272, 102 CrossRef CAS PubMed.
- Y. K. Ong, F. Y. Li, S. P. Sun, B. W. Zhao, C. Z. Liang and T. S. Chung, Chem. Eng. Sci., 2014, 114, 51 CrossRef CAS PubMed.
- L. Shao, X. Q. Cheng, Y. Liu, S. Quan, J. Ma, S. Z. Zhao and K. Y. Wang, J. Membr. Sci., 2013, 430, 96 CrossRef CAS PubMed.
- S. J. Deng, R. Wang, H. J. Xu, X. S. Jiang and J. Yin, J. Mater. Chem., 2012, 22, 10055 RSC.
- S. J. Deng, H. J. Xu, X. S. Jiang and J. Yin, Macromolecules, 2013, 46, 2399 CrossRef CAS.
- B. Li, Y. C. Dong, C. Zou and Y. M. Xu, Ind. Eng. Chem. Res., 2014, 53, 4199 CrossRef CAS.
- A. Yadav, S. Mukherji and A. Garg, Ind. Eng. Chem. Res., 2013, 52, 10063 CrossRef CAS.
- C. S. D. Rodrigues, L. M. Madeira and R. A. Boaventura, Ind. Eng. Chem. Res., 2014, 53, 2412 CrossRef CAS.
- B. E. Tastan, S. E. Karatay and G. Donmez, Water Sci. Technol., 2012, 66, 2177 CrossRef CAS PubMed.
- J. Hu, H. J. Yu, W. Dai, X. Y. Yan, X. Hu and H. Huang, RSC Adv., 2014, 4, 35124 RSC.
- F. Liu, S. Chung, G. Oh and T. S. Seo, ACS Appl. Mater. Interfaces, 2012, 4, 922 CAS.
- R. Gong, J. Ye, W. Dai, X. Yan, J. Hu, X. Hu, S. Li and H. Huang, Ind. Eng. Chem. Res., 2013, 52, 14297 CrossRef CAS.
- M. d. Islam Azharul, I. A. W. Tan, A. Benhouria, M. Asif and B. H. Hameed, Chem. Eng. J., 2015, 270, 187 CrossRef PubMed.
- F. Güzel, H. Saygli, G. A. Saygli and F. Koyuncu, J. Mol. Liq., 2014, 194, 130 CrossRef PubMed.
- T. E. Rufford, D. Hulicova-Jurcakova, K. Khosla, Z. H. Zhu and G. Q. Lu, J. Power Sources, 2010, 195, 912 CrossRef CAS PubMed.
- C. Peng, X. B. Yan, R. T. Wang, J. W. Lang, Y. J. Ou and Q. J. Xue, Electrochim. Acta, 2013, 87, 401 CrossRef CAS PubMed.
- X. L. Wu, T. Wen, H. L. Guo, S. B. Yang, X. K. Wang and A. W. Xu, ACS Nano, 2013, 7, 3589 CrossRef CAS PubMed.
- Y. K. Lv, L. H. Gan, M. X. Liu, W. Xiong, Z. J. Xu, D. Z. Zhu and D. S. Wright, J. Power Sources, 2012, 209, 152 CrossRef CAS PubMed.
- D. Jimenez-Cordero, F. Heras, M. A. Gilarranz and E. Raymundo-Pinero, Carbon, 2014, 71, 127 CrossRef CAS PubMed.
- X. Li, W. Xing, S. P. Zhuo, J. Zhou, F. Li, S. Z. Qiao and G. Q. Lu, Bioresour. Technol., 2011, 102, 1118 CrossRef CAS PubMed.
- S. H. Du, L. Q. Wang, X. T. Fu, M. M. Chen and C. Y. Wang, Bioresour. Technol., 2013, 139, 406 CrossRef CAS PubMed.
- X. J. He, R. C. Li, J. S. Qiu, K. Xie, P. H. Ling, M. X. Yu, X. Y. Zhang and M. D. Zheng, Carbon, 2012, 50, 4911 CrossRef CAS PubMed.
- Q. Wang, J. Yan, Y. B. Wang, T. Wei, M. L. Zhang, X. Y. Jing and Z. J. Fan, Carbon, 2014, 67, 119 CrossRef CAS PubMed.
- T. Katekunlaphan, R. Chalermglin, T. Rukachaisirikul and P. Chalermglin, Biochem. Syst. Ecol., 2015, 57, 152 CrossRef PubMed.
- M. L. Yola, T. Eren, N. Atar and S. Wangc, Chem. Eng. J., 2014, 242, 333 CrossRef CAS PubMed.
- L. Shao, Z. X. W. Y. L. Zhang, Z. X. Jiang and Y. Y. Liu, J. Membr. Sci., 2014, 461, 10 CrossRef CAS PubMed.
- A. Barroso-Bogeat, M. Alexandre-Franco, C. Fernández-González and V. Gómez-Serrano, Energy Fuels, 2014, 28, 4096 CrossRef CAS.
- Á. Sánchez-Sánchez, F. Suárez-García, A. Martínez-Alonso and J. M. D. Tascón, J. Colloid Interface Sci., 2015, 450, 91 CrossRef PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361 CrossRef CAS.
- H. Freundlich, J. Phys. Chem., 1906, 57, 385 CAS.
- M. I. Temkin and V. Pyzhev, Acta Physicochim. URSS, 1940, 12, 327 CAS.
- M. M. Dubinin and L. V. Radushkevich, Dokl. Akad. Nauk SSSR, 1947, 55, 327 CAS.
- R. S. Ribeiro, N. A. Fathy, A. A. Attia, A. M. T. Silva, J. L. Faria and H. T. Gomes, Chem. Eng. J., 2012, 195, 112 CrossRef PubMed.
- M. Ghaedi, F. Mohammdi and A. Ansari, J. Dispersion Sci. Technol., 2015, 36, 652 CrossRef CAS PubMed.
- M. Ahmaruzzaman and R. A. Reza, Environ. Prog. Sustainable Energy, 2014, 34, 724 CrossRef PubMed.
- S. Langergen and B. K. Svenska, K. Sven. Vetenskapsakad. Handl., 1898, 24, 1 Search PubMed.
- Y. S. Ho and G. Mckay, Process Biochem., 1999, 34, 451 CrossRef CAS.
- G. Annadurai, R. S. Juang and D. J. Lee, J. Hazard. Mater., 2002, 92, 263 CrossRef CAS.
- H. I. Chieng, N. Priyanthabc and L. B. L. Lim, RSC Adv., 2015, 5, 34603 RSC.
- M. R. Malekbala, M. A. Khan, S. Hosseinia, L. C. Abdullah and T. S. Y. Choonga, J. Ind. Eng. Chem., 2015, 21, 369 CrossRef CAS PubMed.
- L. Liu, Z. Y. Gao, X. P. Su, X. Chen, L. Jiang and J. M. Yao, ACS Sustainable Chem. Eng., 2015, 3, 432–442 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11568j |
| This journal is © The Royal Society of Chemistry 2015 |