Application of magnetic nanoparticles modified with poly(2-amino thiophenol) as a sorbent for solid phase extraction and trace detection of lead, copper and silver ions in food matrices†
Abstract
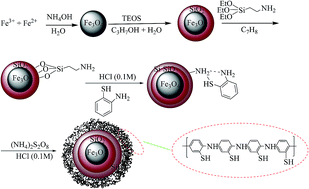
We are introducing magnetic nanoparticles modified with poly(2-amino thiophenol) as a new solid-phase for the extraction of heavy metals ions, including lead(II), copper(II) and silver(I). The synthesized sorbent was characterized by Fourier transform infrared spectrometry, elemental analysis, scanning electron microscopy, energy-dispersive X-ray spectrometry, thermogravimetric analysis and X-ray diffraction analysis. Separation of the synthesized sorbent from the sample solution was simply achieved by applying an external magnetic field. Determination of the extracted ions was performed by flame atomic absorption spectrophotometry (FAAS). Effects of pH value, adsorption and desorption time, type, concentration and volume of the eluent, breakthrough volume, and effect of potentially interfering ions were studied. Under the optimized conditions, the extraction efficiency is >95%, the limits of detection are 2.1, 0.4 and 1.1 ng mL−1 for lead, copper and silver ions, and the adsorption capacities for these ions are 78.2, 68.1 and 52.3 mg g−1, respectively. The data obtained for the adsorption capacity of the sorbent shows the high tendency of the sorbent towards the target ions mentioned. Finally, this sorbent can be used as a simple, rapid, reliable, selective and sensitive method for the determination of trace levels of lead(II), copper(II) and silver(I) in different food samples.

 Please wait while we load your content...
Please wait while we load your content...