Fluorescence sensing and intracellular imaging of Al3+ ions by using naphthalene based sulfonamide chemosensor: structure, computation and biological studies†
Abstract
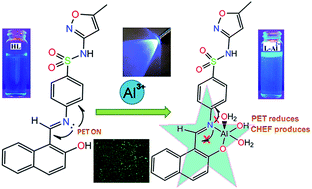
A naphthalene based sulfonamide Schiff base, (E)-4-(((2-hydroxynaphthalen-1-yl)methylene)amino)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (HL) has been found to be a flourescence turn-on probe for selective detection of Al3+ in aqueous system. Structure of the probe has been established by FTIR, 1H NMR, mass spectra and X-ray single crystal study. The probe has shown 24 times flourescence enhancement in presence of Al3+. The limit of detection (LOD) obtained by 3σ method is 33.2 nM. The probable co-ordination environment of L-Al2+ complex has been supported by mass spectral data and DFT computational study. By TD-DFT calculation UV-Vis spectra of HL and L-Al2+ complex has been predicted and that has well correlated with experimental data. Cell imaging study reveals that the probe can be used for the intracellular detection of Al3+ in cultured Vero cell. Antimicrobial activity of HL has also been evaluated and probable mode of binding inside the DHPS cavity has been predicted by docking study. This sensor is unique with reference to two other predecessors because of its bio-compatibility of sulfonamide derivative and nonmutagenicity.

 Please wait while we load your content...
Please wait while we load your content...