Bouquet-like calcium sulfate dihydrate: a highly efficient adsorbent for Congo red dye†
Abstract
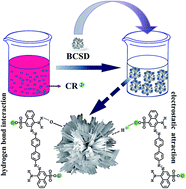
A unique bouquet-like calcium sulfate dihydrate (BCSD) was successfully synthesized from calcium chloride and aluminum potassium sulfate in aqueous sodium carboxymethyl cellulose (CMC) solution by means of a metathesis reaction. The morphology and structure of BCSD were characterized by scanning electron microscopy, powder X-ray diffraction and transmission electron microscopy. The adsorption of different organic dyes from aqueous solutions onto the as-synthesized BCSD was then investigated, taking into account the influences of adsorbent dose (1.0–3.5 g L−1), solution pH (5.0–12.0) and adsorption time. The results indicated that the temperature and agitation rate had no effect on the morphology of the samples. With the increase of CMC concentration from 0.10% to 0.50%, lamellar calcium sulfate dihydrate (LCSD) gradually transformed into rod-like calcium sulfate dihydrate (RCSD), and eventually generated BCSD. The as-prepared BCSD was monoclinic with preferential [021] and [041] orientations. Moreover, BCSD selectively adsorbed Congo red (CR) instead of rhodamine B and methyl orange. The adsorption equilibrium process of CR was an exothermic process and could adequately be described by the Langmuir isotherm model. The calculated maximum adsorption quantity (qmax) was 1224.09 mg g−1 at 303.5 K, which was almost 12 times larger than that onto LCSD (100.80 mg g−1). Additionally, the adsorption process of CR was a multi-step process, and the adsorption kinetics could be described in terms of a pseudo-second-order model. From attenuated total reflectance Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy studies it was concluded that CR was chemisorbed on BCSD. These results indicate that BCSD is a promising candidate in wastewater treatment.

 Please wait while we load your content...
Please wait while we load your content...