Coulomb block effect inducing distinctive dielectric properties in electroless plated barium titanate@silver/poly(vinylidene fluoride) nanocomposites
Abstract
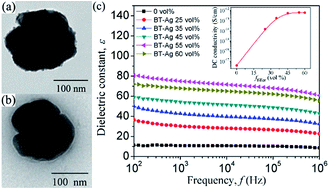
Silver (Ag) nanoparticles with an average diameter of 5–10 nm were dotted on the surface of barium titanate (BaTiO3, BT) by electroless plating. The poly(vinylidene fluoride) (PVDF) nanocomposites filled with BT@Ag hybrid particles were prepared by a solution method. Transmission electron microscopy and X-ray diffraction observations revealed that the Ag nanoparticles were well dotted on the surface of BT nanoparticles, and no phase transformation of BT could be observed. Differential scanning calorimetry results showed that the degree of crystallinity and melting temperature decrease with the increasing content of filler. Breakdown strength of the BT@Ag/PVDF composites as well as BT/PVDF decreased with the increasing volume fraction (ffiller) of fillers. The dielectric constant of BT@Ag/PVDF composites was gradually increased with the increasing ffiller of BT@Ag from 0 to 55 vol%, while a little decrease when ffiller was increased to 60 vol%. It was worth noting that the dielectric constant of BT@Ag/PVDF composites was higher than that of BT/PVDF composites with the same ffiller, and the dielectric loss of BT@Ag/PVDF composites remained low. The direct current conductivity of the BT@Ag/PVDF composites had a significant dependence on the concentration of BT@Ag and stayed in the order of 10−12 S cm−1 when ffiller was more than 35 vol%. These results may be ascribed to the small size of Ag nanoparticles and the Coulomb block effect.

 Please wait while we load your content...
Please wait while we load your content...