Synthesis and evaluation of a glucose attached pyrene, as a fluorescent molecular probe in sugar and non-sugar based micro-heterogeneous media†
Abstract
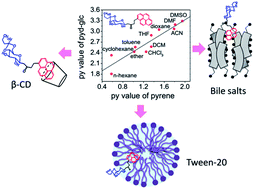
A new fluorescent pyrene–glucose conjugate (pyd-glc), 1-(4,6-O-butylidene-β-D-glucopyranosyl)-4-(1-pyrene)-butan-2-one, has been synthesized by attaching a pyrene molecule to acetal (butylidene) protected glucose via a butane-2-one linker. Detailed photo-physical studies of pyd-glc in homogeneous media have been carried out in both protic and aprotic solvents which lead to a py scale, a solvent polarity scale specific to this molecule, like that of pyrene. The molecule aggregates in water and partitions as a monomer in micro-heterogeneous media. The photo-physical response of pyd-glc has been studied in various sugar and non-sugar based micro-heterogeneous media. Similar to pyrene, this molecule offers multiple fluorescence parameters to investigate the organization of micro-heterogeneous media. The fluorescence parameters of pyd-glc faithfully reflect the state of organization of these anisotropic media.

 Please wait while we load your content...
Please wait while we load your content...