In situ synthesis of g-C3N4/WO3 heterojunction plates array films with enhanced photoelectrochemical performance†
Abstract
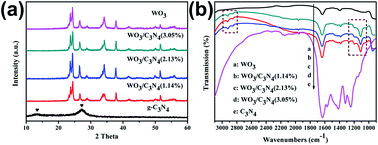
g-C3N4/WO3 heterojunction plate array films with enhanced photoelectrochemical (PEC) performance were successfully synthesized through a combination of hydrothermal and dipping-annealing methods. Urea aqueous solutions were prepared as the precursors to in situ synthesize g-C3N4 nanoparticles on the surface of WO3 platelets. The PEC performances of the photoanodes were investigated by the photocurrent density and incident photon-to-current conversion efficiency (IPCE). As-prepared g-C3N4/WO3 heterojunction films achieved a maximum photocurrent density of 2.10 mA cm−2 at +2.0 V (vs. RHE), which was almost 3-fold higher than that of the pure WO3 film (0.78 mA cm−2) under illumination. And the highest IPCE value increased from 25.1% to 53.1% after the g-C3N4 nanoparticles deposition. The enhanced PEC performance was attributed to the increased carriers density, better electron transport properties, longer electron lifetime and effective charge separation at the interface of heterojunction, which were confirmed by Mott–Schottky and electrochemical impedance spectroscopy (EIS). This study demonstrates that the low-dimensional morphological structure and in situ formation of heterojunction structure are expected to provide a promising photoanode for photoelectric catalysis.

 Please wait while we load your content...
Please wait while we load your content...