Broccoli glucosinolate degradation is reduced performing thermal treatment in binary systems with other food ingredients†
Abstract
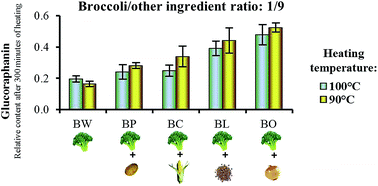
Glucosinolate (GL) stability has been widely studied in different Brassica species. However, the matrix effect determined by the presence of other ingredients occurred in many broccoli-based traditional recipes may affect GL thermal degradation. In this study, the matrix effect on GL thermal degradation was investigated by means of binary systems containing broccoli and another ingredient such as potato, corn starch, lentils protein or onion. Data showed that in binary systems the GL degradation was lower compared to the only-broccoli system, in particular in the broccoli/onion systems. The kinetics of GL degradation in broccoli/onion systems at different ratios showed that the higher the amount of onion, the higher the protective effect and that GL thermal degradation followed a second order model. Finally the possibility that the effect was related to the amount of flavonoids present in onions was ruled out by data obtained using broccoli/onion systems made with three onion varieties having different flavonoid content. This study shows for the first time that the presence of other food ingredients can efficiently reduce GL thermal degradation. The protective effect of onion, often present in the traditional recipes of broccoli soups in many countries, points out that the interaction of different ingredients may not only improve the taste of a dish, but also the healthiness.

 Please wait while we load your content...
Please wait while we load your content...