BF3 bonded nano Fe3O4 (BF3/MNPs): an efficient magnetically recyclable catalyst for the synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives†
Mohammad Abdollahi-Alibeik*,
Ali Moaddeli and
Kianoosh Masoomi
Department of Chemistry, Yazd University, Yazd 89158-13149, Iran. E-mail: abdollahi@yazd.ac.ir; moabdollaho@gmail.com; Fax: +98-35-38210644; Tel: +98-35-31232659
First published on 26th August 2015
Abstract
A simple and efficient procedure for the synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives has been developed by a one-pot three-component reaction of various aldehydes with malononitrile and 3-methyl-1-phenyl-2-pyrazoline-5-one in the presence of BF3/MNPs as a novel nanostructured, heterogeneous and reusable catalyst. In this research, BF3/MNPs nanoparticles were prepared at three calcination temperatures and characterized by various techniques. The characterization and optimization results show that the catalyst with a calcination temperature of 450 °C has the best catalytic activity. The nano-sized magnetite catalyst were recovered by simple separation with an external magnet and reused for several cycles without considerable loss of activity.
1. Introduction
Many conventional liquid inorganic acids, such as HNO3, BF3 and H2SO4 have been replaced by heterogeneous solid acid catalysts in acid-catalyzed organic transformations. Environmental pollution and difficulties in handling and separation of such homogeneous catalysts and also contamination of the products by residual catalyst, greatly restrict their applications from a process and economic point of view. Immobilization of inorganic acid on solid supports is a suitable way to improve the mentioned drawbacks and this way combines high surface area with the additional benefit of relatively facile recovery and regeneration.1Solid-supported catalysts are an important and growing arena in heterogeneous catalysis. Therefore, a key challenge is to use a suitable and stable support with a large surface area to reach high accessibility to maximum active catalytic sites and maximum catalyst loading. Nano-sized solid-supports such as ZrO2,2,3 TiO2,4,5 Al2O3,6 ZnO7 and SiO2 (ref. 8 and 9) have attracted much attention due to their versatile physical properties and also applications in catalysis. However, conventional separation methods for these tiny support particles may become inefficient.
Magnetite nanoparticles are one of the most widely studied materials in multi-disciplinary researches including biotechnology,10 biomedicine,11 magnetic resonance imaging (MRI),12 targeted drug delivery13 and catalysis.14,15 As the catalyst, magnetite has been used in several important commercial processes such as ammonia synthesis,16 water gas shift reaction17 and Fischer–Tropsch reaction,18 which are important routes to get high value intermediates for chemical and petrochemical industries.
Recently, nano-magnetite has found versatile applications as a solid-support for preparation of recyclable catalysts in the development of sustainable methodologies.19 Surface functionalization of magnetic nanoparticles is a well-designed way to bridge the gap between heterogeneous and homogeneous catalysis to increase catalytic activity of MNPs.20–22 Due to its magnetic properties, it is also useful as component of several catalysts and adsorbents for different applications, allowing its separation from medium after reaction.
Fused pyran derivatives represent an important class of compounds which possess high activity profile due to their wide range of biological activities such as antimicrobial,23 antiviral24 and cancer therapy.25 Fused pyrans to pyrazoles as pyranopyrazoles are an important class of heterocyclic compounds. They find applications as biodegradable agrochemicals26 and pharmaceutical ingredients.27,28 The first synthetic method of this nucleus has been reported by Junek and co-workers by the reaction between 3-methyl-1-phenylpyrazolin-5-one and tetracyanoethylene.29 Afterward, various precursors and various acidic30,31 or basic27,32–34 catalysts have been introduced for the synthesis of pyranopyrazoles.
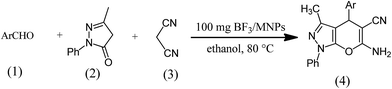
In this research, we supported BF3 on Fe3O4 and bonded it to the surface of support using thermal operations at various temperature as a novel solid acid and magnetically recoverable catalyst for the synthesis of pyranopyrazoles through multi-component reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one, malononitrile and various aromatic aldehydes (Scheme 1).
2. Experimental
2.1. Materials and methods
All chemicals were commercial products. All reactions were monitored by TLC (Thin Layer Chromatography) and all yields refer to isolated products. 1H and 13C NMR spectra were recorded in DMSO-d6 on a Bruker DRX-400 AVANCE (400 MHz for 1H and 100 MHz for 13C) spectrometer. Infrared spectra of the catalysts and reaction products were recorded on a Bruker FT-IR Equinox-55 spectrophotometer in KBr disks. XRD patterns were recorded on a Bruker D8 ADVANCE X-ray diffractometer using nickel filtered Cu Kα radiation (λ = 1.5406 Å). Scanning electron microscopy (SEM) was performed using KYKY-EM3200 instrument. Potentiometric data was collected using pH/mV meter, AZ model 86502-pH/ORP. ICP analysis was performed by VARIAN model Vista-pro instrument.2.2. Preparation of MNPs
Fe3O4–MN was prepared by co-precipitation method as reported.35 In a typical procedure 0.5 M ferrous chloride (10 mL) and 0.5 M ferric chloride ( 20 mL) were mixed in a glass beaker. To this solution, 12 M NH4OH (60 mL) was added drop by drop with continuous stirring. The resulting black precipitate was kept for 2 h. The precipitate was washed three times with deionized water (20 mL) to remove excess NH3.2.3. Preparation of BF3/MNPs
A mixture of MNPs (1 g), toluene (10 mL) and BF3·Et2O (3 mmol) was stirred for 2 h at room temperature. The suspension was separated by centrifuge and washed with toluene (10 mL). The solid was dried in an oven at 120 °C for 1 h and then calcined at 350, 400 or 450 °C for 2 h. The samples were labeled as BF3/MNPs-X where X is the final calcination temperature (Scheme 2).2.4. General procedure for the synthesis of derivatives
A mixture of aryl aldehyde 1 (1 mmol), 3-methyl-1-phenyl-2-pyrazoline-5-one 2 (1 mmol), malononitrile 3 (1 mmol) and BF3/MNPs (100 mg) was stirred in ethanol (5 mL) at 80 °C for mentioned times in Table 2. After completion of the reaction (monitored by TLC), the catalyst was separated from solid product by an external magnet, and product washed with small amounts of water (10 mL) and ethanol (5 mL) then recrystallized from ethanol to give the pure products 4a–j.2.5. Physical and spectroscopic data for selected compounds
3. Results and discussions
3.1. The catalyst characterization
Fig. 1 represents the results of scanning electron microscopy (SEM) in order to investigate the particle size and morphology of the catalysts. The SEM of the MNPs and BF3/MNPs shows spherical nanoparticles with sizes of <100 nm. In the case of BF3/MNPs, partial agglomeration is observed due to BF3 treatment on MNPs surface and also calcination, but this treatment has not dramatically effect on the nanoparticle shapes. To investigate the elemental component of the BF3/MNPs-450, EDX analysis was performed and shown in Fig. 1c. Presence of the Fe and O related to the MNPs is obvious. In addition, EDX analysis shows considerable content of the F. Moreover the loading level of boron on the surface of BF3/MNPs-450 was estimated to be at about 0.5 mmol g−1 with an ICP method and the fluoride contents of BF3/MNPs-450 was estimated to be at about 0.75 mmol g−1 and it was measured by a potentiometric method using a fluoride ion-selective electrode. These results verify presence of B–F species in the catalyst and the obtained B/F molar ratio of 3/2 shows that boron species on the MNPs surface. The B/F molar ratio of 3/2 suggests that a covalent bond between oxygen of Fe3O4 and boron is created due to evolution of HF during calcination.TEM image of the BF3/MNPs-450 is shown in Fig. 2. This image demonstrates nearly uniform size of the particles and spherical shape of them.
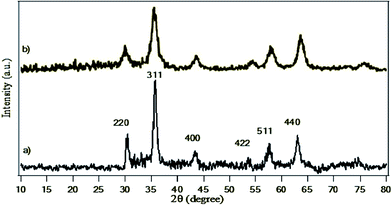
XRD pattern of magnetite nanoparticles is shown in Fig. 3. Both Fe3O4 and BF3/MNPs-450 show diffraction peaks at 2θ = 30.3, 35.6, 43.3, 53.8, 57.4 and 62.9° that are indexed to the crystalline cubic inverse spinel structure of Fe3O4 nanoparticles.
Fig. 4 shows the IR spectra of MNPs and BF3/MNPs at different calcination temperatures over the 400–4000 cm−1 region. As shown in Fig. 4, all the samples show characteristic peaks at 560 and 638 cm−1, which are assigned to Fe–O stretching modes. The peak at 1083 is assigned to C–O (the residue of ether) that is not observed in the calcined samples. Apart from the main peaks of MNPs, there is a wide peak at ∼1400 cm−1, which is assigned to B–O stretching.36 This peak is observed before calcination of the catalyst and also is observed in all calcined samples but with less intensity and partly broadening. Surprisingly, this peak has a larger relative intensity respect to the other calcined samples.
 | ||
| Fig. 4 FT-IR spectra of (a) MNPs and BF3/MNPs (b) before calcination (c) calcination at 350 °C (d) calcination at 400 °C (e) calcination at 450 °C. | ||
The catalyst acidity characters, including the acidic strength and the total number of acid sites were determined by potentiometric titration. According to this method, the initial electrode potential (Ei) indicates the maximum acid strength of the surface sites.37 Therefore, a suspension of the catalyst in acetonitrile was potentiometrically titrated with a solution of 0.02 M n-butylamine. As shown in Fig. 5, BF3/MNPs-450 displays higher strength than the MNPs.
Fig. 6 shows the magnetization versus applied field of the catalyst that was obtained by VSM. The saturation magnetization value was measured to be ∼60 emu g−1 for Fe3O4 and ∼50 emu g−1 for BF3/MNPs-50. The results show that surface modification of MNPs has insignificance effect on the magnetic properties of MNPs (Fig. 7).
After characterization of the prepared catalysts, to determination of the best catalytic activity, they have been used in the multi-component reaction of 4-chlorobenzaldehyde, malononitrile and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one as model reaction. The reaction was optimized for various parameters such as temperature, solvent and catalyst loading. We first, investigated effect of the calcination temperature on the catalytic behavior of prepared samples. In the presence of an equal amount of the catalyst (100 mg), BF3/MNPs-450 show the better catalytic activity in term of the yield of desired product and time of completion of model reaction in ethanol as the reaction solvent. Therefore, other reaction parameters has been optimized in the presence of BF3/MNPs-450. To optimize the catalyst amount, the model reaction was performed in the presence of various amounts of the catalyst and according to the obtained results (Table 1, entries 1–4) 100 mg of the catalyst was chosen as the best catalyst amount.
| Entry | Catalyst | Catalyst amount (mg) | Timeb (min) | Yieldc (%) |
|---|---|---|---|---|
| a All reactions were carried out with 4-chlorobenzaldehyde (1 mmol), malononitrile (1.1 mmol), 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (1 mmol), ethanol (5 mL) and BF3/MNPs-X as the catalyst at 80 °C.b Reaction times is monitored by TLC.c Isolated yield. | ||||
| 1 | BF3/MNPs-450 | 50 | 30 | 86 |
| 2 | BF3/MNPs-450 | 75 | 20 | 89 |
| 3 | BF3/MNPs-450 | 100 | 15 | 96 |
| 4 | BF3/MNPs-450 | 125 | 25 | 79 |
| 5 | BF3/MNPs-450 | 100 | 60 | 85 |
| 6 | BF3/MNPs-450 | 100 | 60 | 83 |
| 7 | MNPs | 100 | 180 | 40 |
| 8 | BF3·Et2O | 7 | 35 | 86 |
The effect of solvent was also investigated by performing the model reaction in the presence of 100 mg catalyst in various solvents (Table 2, entries 1–5). Among them, ethanol was found to be the best solvent in reflux condition (80 °C) in terms of the reaction time and yield of desired product (Table 2, entry 1). The model reaction in the presence of ethanol as the solvent was also performed at the lower temperature (70 °C) and also the less yield and longer reaction time was obtained (Table 2, entry 6). To investigate efficiency of the support on the catalytic activity of BF3 the model reaction was performed in the presence of BF3·Et2O (7 mg, equal to loading amount of boron on the 100 mg catalyst) and results shows lower activity than BF3/MNPs-450. MNPs was also applied as the catalyst in the model reaction and results show that MNPs lacks catalytic activity in this type of reaction.
| Entry | Catalyst amount (mg) | Solvent | Temp. (°C) | Timeb (min) | Yieldc (%) |
|---|---|---|---|---|---|
| a All reactions were carried out with 4-chlorobenzaldehyde (1 mmol), malononitrile (1.1 mmol), 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (1 mmol) and BF3/MNPs-450 as the catalyst.b Reaction times is monitored by TLC.c Isolated yield. | |||||
| 1 | 100 | EtOH | 80 | 15 | 96 |
| 2 | 100 | H2O | 100 | 45 | 60 |
| 3 | 100 | DMF | 80 | 15 | 90 |
| 4 | 100 | THF | 65 | 15 | 53 |
| 5 | 100 | MeOH | 65 | 25 | 65 |
| 6 | 100 | EtOH | 70 | 30 | 80 |
Thereafter, the above optimized reaction conditions were explored for the synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives and the results are summarized in Table 3. As exemplified in Table 3, this protocol is rather general for a wide variety of electron-rich as well as electron-deficient aromatic aldehydes.
| Entry | Substrate 1 | Product 4 | Timeb (min) | Yieldc (%) | Mp (°C) (ref.) |
|---|---|---|---|---|---|
| a All reactions were carried out with 4-chlorobenzaldehyde (1 mmol), malononitrile (1.1 mmol), 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (1 mmol) in ethanol (5 mL) and BF3/MNPs-450 as the catalyst at 80 °C.b Reaction times is monitored by TLC.c Isolated yield. | |||||
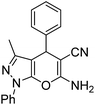
| a |  |
 |
15 | 96 | 178–180 (ref. 38) |
| b |  |
 |
5 | 88 | 172–174 (ref. 38) |
| c |  |
 |
10 | 90 | 192–194 (ref. 38) |
| d |  |
 |
15 | 85 | 172–173 (ref. 38) |
| e |  |
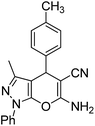 |
60 | 93 | 175–177 (ref. 38) |
| f |  |
 |
15 | 87 | 160–161 (ref. 39) |
| g |  |
 |
15 | 84 | 175–177 (ref. 40) |
| h |  |
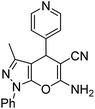 |
60 | 90 | 195–197 (ref. 27) |
| i |  |
 |
20 | 88 | 213–214 (ref. 38) |
| j |  |
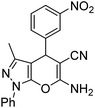 |
15 | 91 | 190–191 (ref. 40) |
A plausible mechanism for the synthesis of pyranopyrazoles catalyzed by BF3/MNPs is explained in Scheme 3.
Reusability of the catalyst was investigated in the model reaction under the optimized reaction conditions. The catalyst was separated from the model reaction with an external magnet easily and reused four times with negligible loss of the catalytic activity (Table 4). Partial loss of activity may be due to blockage of active sites of the catalyst and/or partial leaching of boron from the catalyst.
| Fresh catalyst | First cycle | Second cycle | Third cycle | Fourth cycle | |
|---|---|---|---|---|---|
| Time (min) | 15 | 15 | 15 | 15 | 15 |
| Yield (%) | 95 | 90 | 88 | 85 | 80 |
A comparative study of this work with other methods has performed. Table 5 presents other reported methods for the synthesis of pyranopyrazole derivatives. Although Table 5 contains various methods such as four-component synthesis of pyranopyrazoles, we can say that our method is comparable with other reported method in terms of yield and reaction time. The most significance of our method is use of the magnetite heterogeneous solid acid catalyst with good catalyst recoverability and ease of separation from reaction media. In addition, use of commercial available precursors, green solvent and easy work-up make this method attractive for the synthesis of pyranopyrazole derivatives.
4. Conclusion
In conclusion, we prepared BF3/MNPs as a novel magnetite recoverable catalyst and it has been characterized using various techniques such as SEM, TEM, EDX, XRD, FT-IR and VSM. In this study immobilization of BF3 on the MNPs was performed through thermal treatment (calcination) and it was observed that calcination temperature have important effect on the catalysis activity of the catalyst. The catalytic activity of the prepared catalysts at the different calcination temperature was investigated in the synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives through one-pot multi-component reaction of aldehyde, malononitrile and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one and BF3/MNPs-450 showed better catalytic activity respect to the other samples. From the synthetic method point of view, use of a reusable catalyst, moderate to good yield of products, he simple experimental procedure, easy workup and ease of the magnetite catalyst recovery make this method attractive for the synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives.Acknowledgements
We are thankful to the Yazd University Research Council for partial support of this work.References
- T. Vijai Kumar Reddy, G. Sandhya Rani, R. B. N. Prasad and B. L. A. Prabhavathi Devi, RSC Adv., 2015, 5, 40997–41005 RSC.
- Y. R. Girish, K. S. Sharath Kumar, U. Muddegowda, N. K. Lokanath, K. S. Rangappa and S. Shashikanth, RSC Adv., 2014, 4, 55800–55806 RSC.
- M. Abdollahi-Alibeik and E. Shabani, J. Iran. Chem. Soc., 2014, 11, 351–359 CrossRef CAS.
- H. Tian, X. L. Zhang, J. Scott, C. Ng and R. Amal, J. Mater. Chem. A, 2014, 2, 6432 CAS.
- N. D. Tran, M. Besson and C. Descorme, New J. Chem., 2011, 35, 2095–2104 RSC.
- S. Fuentes, N. E. Bogdanchikova, G. Diaz, M. Peraaza and G. C. Sandoval, Catal. Lett., 1997, 47, 27–34 CrossRef CAS.
- M. Hosseini-Sarvari, Z. Razmi and M. M. Doroodmand, Appl. Catal., A, 2014, 475, 477–486 CrossRef CAS PubMed.
- M. Abdollahi-Alibeik and A. Moaddeli, New J. Chem., 2015, 39, 2116–2122 RSC.
- M. Abdollahi-Alibeik and A. Moaddeli, RSC Adv., 2014, 4, 39759–39766 RSC.
- T. Matsunaga, in Iron Biominerals, ed. R. Frankel and R. Blakemore, Springer, US, 1991, ch. 6, pp. 79–95, DOI:10.1007/978-1-4615-3810-3_6.
- R. K. Singh, T. H. Kim, K. D. Patel, J. C. Knowles and H. W. Kim, J. Biomed. Mater. Res., Part A, 2012, 100, 1734–1742 CrossRef PubMed.
- C. Y. Haw, F. Mohamed, C. H. Chia, S. Radiman, S. Zakaria, N. M. Huang and H. N. Lim, Ceram. Int., 2010, 36, 1417–1422 CrossRef CAS PubMed.
- T. Neuberger, B. Schöpf, H. Hofmann, M. Hofmann and B. von Rechenberg, J. Magn. Magn. Mater., 2005, 293, 483–496 CrossRef CAS PubMed.
- S. Ko and J. Jang, Angew. Chem., Int. Ed., 2006, 45, 7564–7567 CrossRef CAS PubMed.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- A. Baiker, H. Baris and R. Schlögl, J. Catal., 1987, 108, 467–479 CrossRef CAS.
- C. R. F. Lund, J. E. Kubsh and J. A. Dumesic, in Solid State Chemistry in Catalysis, ACS Symposium Series, 1985, vol. 279, pp. 313–338 Search PubMed.
- K. R. P. M. Rao, F. Huggins, V. Mahajan, G. Huffman and V. U. S. Rao, Hyperfine Interact., 1994, 93, 1745–1749 CrossRef CAS.
- K. Debnath, K. Singha and A. Pramanik, RSC Adv., 2015, 5, 31866–31877 RSC.
- A. Khalafi-Nezhad, M. Nourisefat and F. Panahi, RSC Adv., 2014, 4, 22497 RSC.
- M. Kotani, T. Koike, K. Yamaguchi and N. Mizuno, Green Chem., 2006, 8, 735 RSC.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371–3393 RSC.
- N. J. Thumar, ARKIVOC, 2010, 2009, 363 CrossRef.
- P. W. Smith, S. L. Sollis, P. D. Howes, P. C. Cherry, I. D. Starkey, K. N. Cobley, H. Weston, J. Scicinski, A. Merritt, A. Whittington, P. Wyatt, N. Taylor, D. Green, R. Bethell, S. Madar, R. J. Fenton, P. J. Morley, T. Pateman and A. Beresford, J. Med. Chem., 1998, 41, 787–797 CrossRef CAS PubMed.
- J. Skommer, D. Wlodkowic and J. Pelkonen, Exp. Hematol., 2006, 34, 463–474 CrossRef CAS PubMed.
- H. V. Chavan, S. B. Babar, R. U. Hoval and B. P. Bandgar, Bull. Korean Chem. Soc., 2011, 32, 3963–3966 CrossRef CAS.
- G. Vasuki and K. Kumaravel, Tetrahedron Lett., 2008, 49, 5636–5638 CrossRef CAS PubMed.
- M. Babaie and H. Sheibani, Arabian J. Chem., 2011, 4, 159–162 CrossRef CAS PubMed.
- H. Junek and H. Aigner, Chem. Ber., 1973, 106, 914–921 CrossRef CAS PubMed.
- S. Pal, M. N. Khan, S. Karamthulla, S. J. Abbas and L. H. Choudhury, Tetrahedron Lett., 2013, 54, 5434–5440 CrossRef CAS PubMed.
- M. A. Zolfigol, M. Tavasoli, A. R. Moosavi-Zare, P. Moosavi, H. G. Kruger, M. Shiri and V. Khakyzadeh, RSC Adv., 2013, 3, 25681 RSC.
- B. Maheshwar Rao, G. N. Reddy, T. V. Reddy, B. L. A. P. Devi, R. B. N. Prasad, J. S. Yadav and B. V. S. Reddy, Tetrahedron Lett., 2013, 54, 2466–2471 CrossRef CAS PubMed.
- Y. Zou, Y. Hu, H. Liu and D. Shi, ACS Comb. Sci., 2012, 14, 38–43 CrossRef CAS PubMed.
- S. H. S. Azzam and M. A. Pasha, Tetrahedron Lett., 2012, 53, 6834–6837 CrossRef CAS PubMed.
- R. Ghosh, L. Pradhan, Y. P. Devi, S. S. Meena, R. Tewari, A. Kumar, S. Sharma, N. S. Gajbhiye, R. K. Vatsa, B. N. Pandey and R. S. Ningthoujam, J. Mater. Chem., 2011, 21, 13388 RSC.
- M. Abdollahi-Alibeik and A. Rezaeipoor-Anari, Catal. Sci. Technol., 2014, 4, 1151–1159 CAS.
- L. R. Pizzio, P. G. Vázquez, C. V. Cáceres and M. N. Blanco, Appl. Catal., A, 2003, 256, 125–139 CrossRef CAS.
- H. S. Sohal, A. Goyal, R. Sharma, R. Khare and S. Kumar, Eur. J. Chem., 2013, 4, 450–453 CrossRef CAS.
- M. Farahi, B. Karami, I. Sedighimehr and H. M. Tanuraghaj, Chin. Chem. Lett., 2014, 25, 1580–1582 CrossRef CAS PubMed.
- K. Niknam, N. Borazjani, R. Rashidian and A. Jamali, Chin. J. Catal., 2013, 34, 2245–2254 CrossRef CAS.
- S. Paul, K. Pradhan, S. Ghosh, S. K. De and A. R. Das, Tetrahedron, 2014, 70, 6088–6099 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11343a |
| This journal is © The Royal Society of Chemistry 2015 |