Umbelliprenin and lariciresinol isolated from a long-term-used herb medicine Ferula sinkiangensis induce apoptosis and G0/G1 arresting in gastric cancer cells†
Lijing Zhanga,
Jianyong Sia,
Guangzhi Lia,
Xiaojin Lib,
Leilei Zhanga,
Li Gaoa,
Xiaowei Huoa,
Dongyu Liua,
Xiaobo Suna and
Li Cao*a
aInstitute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College, 151# Malianwa North Road, Haidian District, Beijing, 100193, China. E-mail: lcao@implad.ac.cn; Fax: +86-010-57833222; Tel: +86-010-57833222
bXinjiang Institute of Chinese Materia Medica and Ethnodrug, Urumqi, Xinjiang Province 830002, China
First published on 15th October 2015
Abstract
Effective chemicals isolated from folk medicine are commonly used in the treatment of cancer in Asian countries like China and India. Ferula sinkiangensis K. M. Shen is a traditional herb medicine used for treating stomach disorders in Xinjiang District of China for thousands of years. Here, we showed that the growth inhibition effects of seven compounds first isolated from the seeds of this herb in human gastric cancer cells and human normal gastric epithelium cells. Furthermore, we characterized the mechanism of the antiproliferation effects on gastric cancer cells of the two most specific and effective compounds: umbelliprenin (UM) and lariciresinol (LA). Annexin V/PI staining demonstrated that UM and LA induce apoptosis in gastric cancer AGS cells. Loss of mitochondrial membrane potential, upregulation of proapoptotic protein BAX, and activation of caspase 3 and PARP suggested that UM and LA caused the activation of the mitochondrial apoptosis pathway. Cell cycle analysis showed that UM and LA arrest cell cycle at G0/G1 phase. Western blot results showed that the expression of P53, P27, P16 and Rb proteins increased, while the expression of cyclin D, cyclin E, Cdk4 and Cdk2 decreased in cancer cells. Overall, these data provided evidence that UM and LA have the potential to be used in cancer therapy.
Introduction
Gastric cancer is characterized by high mortality rates, and one of the most common malignant cancers worldwide.1 The median survival of patients with this metastatic disease is less than one year.2 The current treatment therapy for gastric cancer is chemotherapy, but because the side effects are severe, its application is limited, and effective agents are urgently needed to improve the prognoses of patients. Although several agents are under clinical evaluation for gastric cancer such as trastuzumab and cetuximab,3,4 the effective response rate of gastric cancer patients to these treatments is low (only 30–40%).5 Recently, natural products for gastric cancer treatment therapy have obtained increasing attention.6,7 F. sinkiangensis was originally described in the “Medica of the Tang Dynasty”. As a traditional folk medicine used for the treatment of stomach disorders in Xinjiang District of China for thousands of years, the potential value of this herb for treating gastric cancer could not be ignored.8 Although there have been studies on the chemical composition and anti-inflammation activity of this herb,9,10 the effective components and mechanism in gastric cancer treatment are still not clear. Our previous studies have found that a petroleum ether extract of the seeds showed antitumor activity (unpublished data). Many compounds, including steroidal esters and lignin, have been isolated from the seeds.11,12 In the present study, we first screened these compounds for the growth inhibition effect in gastric cancer cells, and then characterized the possible mechanism. We found that UM and LA were the two most cytotoxic compounds towards gastric cancer cells, and the least cytotoxic to normal gastric epithelial cells, compared with other compounds. In addition, the two compounds induced apoptosis and cell cycle arrest in gastric cancer cells.Materials and methods
Plant material
The seeds of F. sinkiangensis were collected from Yili state, Xinjiang Uygur Autonomous Region of China, in July 2008, and were identified by Professor Xiaojin Li. A voucher specimen (No. AP21020720) was deposited in the Xinjiang Institute of Chinese Materia Medica and Ethnodrug.Test compounds
The seeds of F. sinkiangensis were crushed and refluxed with 95% EtOH for three times, 2 h for each extraction. Then combined the EtOH extracts, evaporated under reduced pressure to yield residue, suspended in water and then partitioned using petroleum ether and dichloromethane. The dichloromethane extract was further fractionated into ten fractions (A–J) using silica gel chromatography with CHCl3–MeOH (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0
1 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Fraction B was subjected to silica gel column chromatography using a Sephadex LH-20 column (2.5 × 150 cm) eluting with MeOH, and ten fractions were obtained. Fraction B-4 and fraction B-6 were purified by semi-preparative HPLC to obtain compound 1 (tR = 19 min), compound 2 (tR = 21 min); fraction B-9 was subjected to silica gel column chromatography eluting with CHCl3–MeOH (20
1, v/v). Fraction B was subjected to silica gel column chromatography using a Sephadex LH-20 column (2.5 × 150 cm) eluting with MeOH, and ten fractions were obtained. Fraction B-4 and fraction B-6 were purified by semi-preparative HPLC to obtain compound 1 (tR = 19 min), compound 2 (tR = 21 min); fraction B-9 was subjected to silica gel column chromatography eluting with CHCl3–MeOH (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 0
1 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and 12 fractions were obtain (fraction B-9-1 to B-9-12). Fraction B-9-4 was eluted with CHCl3–MeOH–H2O (7.5
1, v/v), and 12 fractions were obtain (fraction B-9-1 to B-9-12). Fraction B-9-4 was eluted with CHCl3–MeOH–H2O (7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by preparative scale chromatography and compound 3 was obtained (Rf = 0.6). Then using a MeOH–H2O (47
1) by preparative scale chromatography and compound 3 was obtained (Rf = 0.6). Then using a MeOH–H2O (47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53) system to obtain compound 4 (tR 23 min) and compound 5 (tR 33 min) from fraction B-9-7. Finally, a MeOH–H2O (52
53) system to obtain compound 4 (tR 23 min) and compound 5 (tR 33 min) from fraction B-9-7. Finally, a MeOH–H2O (52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48) system was used to obtain compound 6 (tR 33.7 min) and compound 7 (tR 37.6 min) from fraction B-9-11. These compounds were identified by spectra methods (UV, IR, MS and NMR) and purified by HPLC (purity > 90%). And compound 1 to compounds 7 were (7,8-cis-8,8′-trans)-2-4-dihydroxyl-3,5-dimethoxy-lariciresinol (C20H24O6), lehmannolol (C24H32O4), arctigenin (C21H24O6), quercetin (C15H10O7), macrathoin F (C26H26O12), umbelliprenin (C24H30O3), and lariciresinol (C20H24O6). The chromatographic profiles of UM and LA are shown in ESI.† These compounds were dissolved as stock solutions in dimethyl sulfoxide (DMSO) and subjected to serial dilution with medium before use so the final concentration of DMSO was less than 1% (v/v).
48) system was used to obtain compound 6 (tR 33.7 min) and compound 7 (tR 37.6 min) from fraction B-9-11. These compounds were identified by spectra methods (UV, IR, MS and NMR) and purified by HPLC (purity > 90%). And compound 1 to compounds 7 were (7,8-cis-8,8′-trans)-2-4-dihydroxyl-3,5-dimethoxy-lariciresinol (C20H24O6), lehmannolol (C24H32O4), arctigenin (C21H24O6), quercetin (C15H10O7), macrathoin F (C26H26O12), umbelliprenin (C24H30O3), and lariciresinol (C20H24O6). The chromatographic profiles of UM and LA are shown in ESI.† These compounds were dissolved as stock solutions in dimethyl sulfoxide (DMSO) and subjected to serial dilution with medium before use so the final concentration of DMSO was less than 1% (v/v).
Reagents and antibodies
Dulbecco's Modified Eagle's Medium (DMEM), Ham's F12 medium, trypsin, penicillin, streptomycin, fetal bovine serum (FBS) were purchased from Gibco (CA, USA), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), DCFH-DA, DMSO, Hoechst 33342, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1), RNase A, propidium iodide (PI) and trypan blue were purchased from Sigma-Aldrich (MO, USA). The annexin V-FITC apoptosis detection kit was obtained from KeyGEN Biotech (Jiangsu, China). Antibodies against Bax, Bcl-2, cleaved PARP, cleaved caspase-3, cyclin D1, cyclin E, Cdk4, Cdk2, P16 and P27 were purchased from Santa Cruz Biotechnology (CA, USA). Antibodies against Rb and β-actin were obtained from Cell Signaling Technology (MA, USA). The cECL Western Blot Kit was obtained from CoWin Biotech (Beijing, China). All the chemical reagents were of the highest grade.Cell culture
The human gastric carcinoma cell line AGS and the human prostate carcinoma cell line PC3 were cultured in Ham's F12 medium containing 10% FBS, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin at 37 °C with 5% CO2. The human normal gastric epithelial cell line GES-1, human gastric cancer cell line BGC-823, human cervical carcinoma cell line HeLa and human lung cancer cell line A549 were cultured in DMEM supplemented with 10% FBS, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin under the same conditions. Cells were passaged at least three times before being used in experiments.Animals
Five-week-old male BALB/c nude mice were purchased from Vital River Laboratories (Beijing, China) and maintained on a 12 h light/dark cycle in a regulated environment (25 ± 2 °C), with free access to water and food. The animal protocol was approved by the Animal Ethics Committee at the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences.Cell viability and cytotoxicity assays
MTT assay was used to determine cell viability. Cells were seeded in triplicate in 96-well plates and cultured at 37 °C for 24 h. The cells were treated with compounds in various concentrations (DMSO, 6.25, 12.5, 25, 50, 100 μM). After 24 or 48 h treatment, 10 μL MTT was added (5 mg mL−1) to each well and incubated for another 4 h. The medium was then removed and 150 μL DMSO was added. The absorbance was measured at 570 nm using a Microplate Reader (Bio Tek, USA). Cell viability was expressed as the ratio of surviving cells in each group to cells in control group.Trypan blue exclusion was used to examine the number of dead cells in each group. AGS cells and GES-1 cells were plated in 24-well plates for 24 h and then treated with UM or LA (DMSO, 6.25, 12.5, 25, 50, 100 μM) for 24 h. After harvesting, the cells were suspended in phosphate-buffered saline (PBS) and mixed with 0.4% trypan blue dye solution. The numbers of viable cells and dead cells were counted using light microscope.
Hoechst 33342 and AO/EB staining
AGS cells were cultured in 96-well plates and treated with UM (0, 13.67, 27.34, 54.58 μM) or LA (0, 20.82, 41.62, 83.24 μM) for 24 h. After washing with PBS, cells were stained with Hoechst 33342 for 20 min or stained for 5 min with AO/EB. Nuclear morphology changes were observed using Image Xpress Micro imaging system (Molecular Devices, USA).Apoptosis analysis
UM and LA induced apoptosis in AGS cells were detected using annexin V-FITC/PI apoptosis staining by flow cytometry. Cells were plated and treated with UM (0, 13.67, 27.34, 54.58 μM) or LA (0, 20.82, 41.62, 83.24 μM) for 24 h. After harvesting and washing twice with cold PBS, the cells were incubated with annexin V and PI in binding buffer at room temperature for 30 min in the dark. Stained cells were detected and analyzed using FACS Calibur flow cytometry (Becton Dickinson, USA).28 Apoptotic rates were reported as the percentage of apoptotic cells among total cells.Detection of reactive oxygen species
ROS production was evaluated by the level of hydrogen peroxide produced using DCFH-DA by flow cytometry. AGS cells were seeded in 6-well plates and treated with UM (0, 13.67, 27.34, 54.58 μM) or LA (0, 20.82, 41.62, 83.24 μM) for 24 h. The cells were then harvested and incubated with 10 μM of DCFH-DA in serum-free medium for 30 min in the dark at 37 °C. After washing twice with cold PBS, the cells were analyzed by FACS Calibur flow cytometry to measure ROS levels.Mitochondrial membrane potential measurements (ΔΨm)
Changes in mitochondrial membrane potential after treatments were measured by flow cytometry and Image Xpress Micro imaging system (Molecular Devices, USA) using JC-1. Cells (1 × 106) were treated with UM (0, 13.67, 27.34, 54.58 μM) or LA (0, 20.82, 41.62, 83.24 μM) for 24 h. Cells were then harvested and incubated with JC-1 (5 μM) for 30 min in the dark at 37 °C. After washing twice with PBS, the cells were analyzed by flow cytometry and observed using Image Xpress Micro imaging system.Cell cycle analysis
Cell cycle distribution was measured by staining DNA with PI. Cells (1 × 106) were seeded in 6-well plates and treated with UM (0, 13.67, 27.34, 54.58 μM) or LA (0, 20.82, 41.62, 83.24 μM) for 24 h. Then cells were harvested and fixed with 70% ethanol overnight at −20 °C. After washing twice with PBS, the cells were treated with RNase A for 20 min and then stained with PI (50 mg L−1) for 10 min in the dark29 at room temperature. The distribution of each phase in the cell cycle measured by DNA content was detected using FACS Calibur flow cytometry and analyzed by ModFit LT 4.0 software.Western blot
AGS cells were exposed to UM (0, 13.67, 27.34, 54.58 μM) or LA (0, 20.82, 41.62, 83.24 μM) for 24 h. After collection, cells were lysed in lysis buffer and protein concentrations were determined by the BCA method. Protein samples were separated by SDS-PAGE and electrically transferred onto PVDF membranes. After blocking with 5% non-fat milk solution for 1 h, the membranes were incubated with primary antibody at 4 °C overnight. Later, the primary antibody was washed with TBST and incubated with secondary antibody at room temperature for 1 h. Protein bands were visualized by ECL and the levels of β-actin for each sample were used as a normalizing control.Effects of UM and LA in tumor xenograft models
Mice were inoculated subcutaneously with 1.0 × 106 BGC-823 human gastric cancer cells on the right flank. The mice were randomized to six groups of 8 mice per group the next day. Tumor growth was monitored and tumor size was measured every day. When the tumor volume reached approximately 0.3 mm in diameter, drug administration was initiated. UM and LA were diluted in 0.9% NaCl to the final concentration of 10 mg kg−1 or 20 mg kg−1 in 200 μL solution and administered to each mouse. UM and LA solutions were administered twice a day for 12 days. Mice were then euthanized and tumors were excised and weighed. Tumor inhibition rate = (average weight of control group − average weight of treated group)/average weight of control group × 100%.Statistical analysis
All data were analyzed by software using IBM SPSS statistics 19. Statistical significance between groups was defined as *p < 0.05 and **p < 0.01. Results were expressed as mean ± SD.Results
UM and LA preferentially inhibit the growth and induce the death of human gastric cancer AGS cells
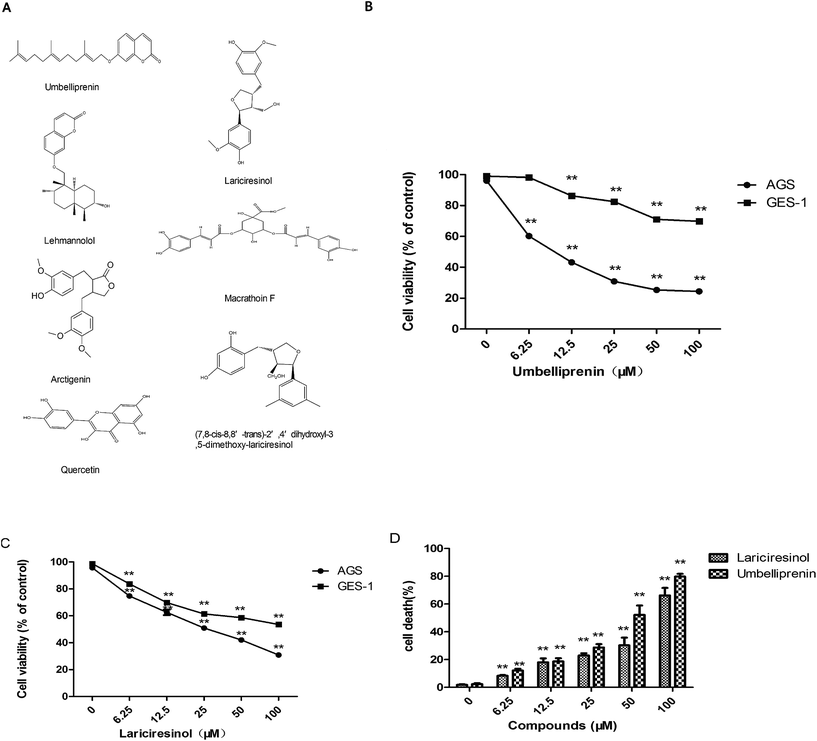
First we studied the anti-proliferative effects of seven compounds isolated from the seeds of F. sinkiangensis (Fig. 1a) against four human commonly observed cancer cell lines: stomach (AGS), cervix (HeLa), lung (A549), prostate (PC3) cancer and human gastric epithelial cell line GES-1. The concentrations resulting in 50% growth inhibition (IC50) were listed in Table 1. The IC50 values for the seven compounds varied for each cell line. A comparison of IC50 values showed that umbelliprenin (UM) and lariciresinol (LA) were the most anti-proliferative compounds against AGS gastric cancer cell line. In addition, UM and LA were less cytotoxic to GES-1 cells compared with AGS cells (Fig. 1b). Therefore, we chose UM and LA for further investigation.| Compounds | IC50a (μM) | ||||
|---|---|---|---|---|---|
| GES-1 | AGS | HeLa | A549 | PC3 | |
| a IC50 is the concentration of compound causing 50% growth inhibition for each cell line. The results represent the mean values of three independent tests. | |||||
| Umbelliprenin | 109.17 ± 2.07 | 13.67 ± 1.73 | 75.83 ± 2.66 | 121.53 ± 4.41 | 88.27 ± 3.76 |
| Lariciresinol | 91.98 ± 1.65 | 20.82 ± 2.86 | 91.36 ± 4.24 | 71.28 ± 2.48 | 104.69 ± 3.45 |
| Quercetin | 44.51 ± 2.29 | 37.62 ± 2.23 | 25.05 ± 2.65 | 52.54 ± 1.59 | 48.87 ± 3.42 |
| Macrathoin F | 62.78 ± 1.01 | 68.31 ± 1.55 | 119.31 ± 3.73 | 77.35 ± 1.72 | 95.69 ± 4.12 |
| Arctigenin | 67.82 ± 1.86 | 87.76 ± 3.67 | 65.23 ± 1.86 | 102.58 ± 3.93 | 54.43 ± 1.91 |
| (7,8-cis-8,8′-trans)-2′,4′-Dihydroxyl-3,5-dimethoxy-lariciresinol | 112.92 ± 3.51 | 89.01 ± 3.14 | 152.84 ± 1.83 | 167.29 ± 4.22 | 118.68 ± 4.83 |
| Lehmannolol | 79.53 ± 2.97 | 156.76 ± 3.78 | 182.38 ± 4.75 | >200 | 187.11 ± 4.91 |
Trypan blue dye exclusion was used to further evaluate the cytotoxicity of the two compounds in AGS cells. Cells were exposed to various concentrations of the two chemicals for 24 h. Fig. 1c showed the increase of dead cells. Together, these data suggested that UM and LA can preferentially inhibit the growth and induce the death of AGS cells while being less cytotoxic to GES-1 cells.
The effects of UM and LA on morphological changes in AGS cells
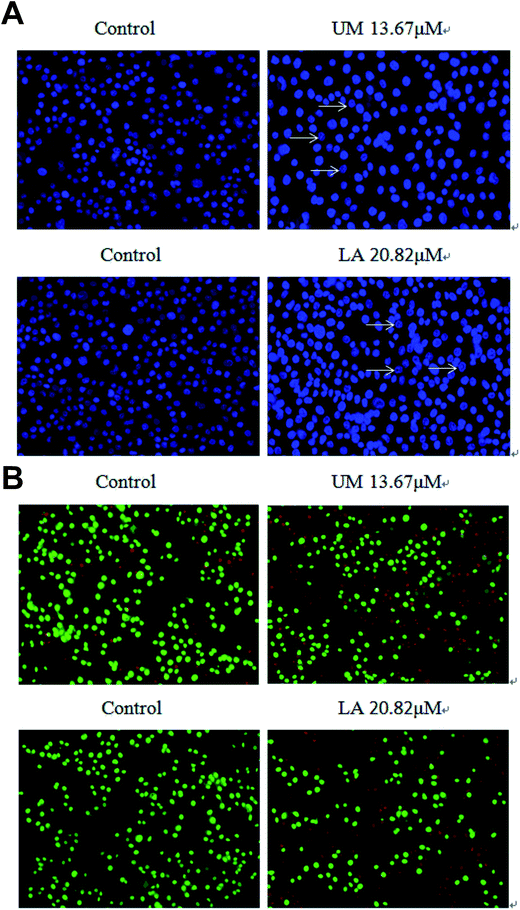
To elucidate whether UM and LA inhibited AGS cell growth by inducing apoptosis, we used Hoechst 33342 and AO/EB staining to study the number of apoptotic cells. Based on IC50 values, we chose 13.67 μM of UM and 20.82 μM of LA and higher concentrations as treatments for AGS cells. After Hoechst 33342 staining, typical morphological changes of apoptosis such as condensed chromatin and apoptotic bodies were observed when cells were exposed to both compounds (Fig. 2a). In contrast, control cells exhibited round nuclei and chromatin were well distributed. After AO/EB staining, apoptosis cells were observed as orange nuclei (Fig. 2b). These observations indicated that the proliferation inhibition effect of UM and LA may be related to apoptosis induction.UM and LA induce apoptosis in AGS cells
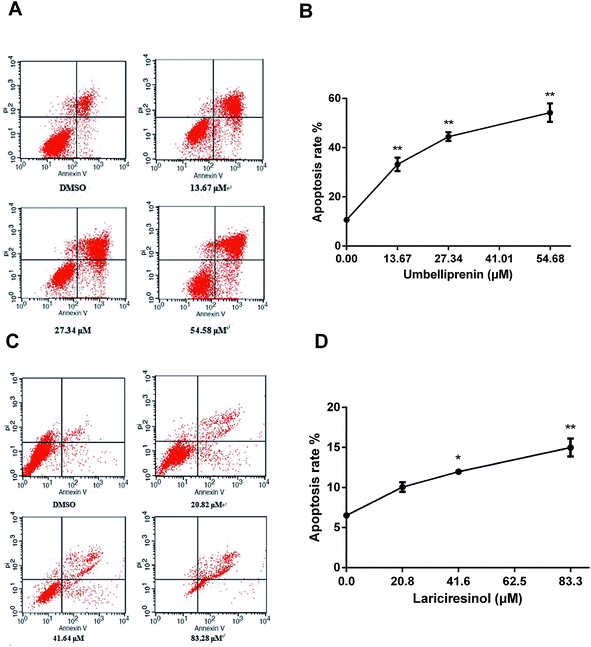
To further characterize the apoptosis process of AGS cells induced by UM and LA, we assessed the numbers of apoptotic cells using annexin V-FITC/PI apoptosis staining. After 24 h exposure to UM, the population of apoptotic cells (AV+/PI− plus AV+/PI+) increased in a dose-dependent manner (Fig. 3a). The cellular apoptotic rates were lower when exposed to LA (Fig. 3b). These results indicate that UM is more effective than LA in the induction of apoptosis in AGS gastric cancer cells.UM and LA induce the decrease of mitochondrial membrane potential (MMP) in AGS cells
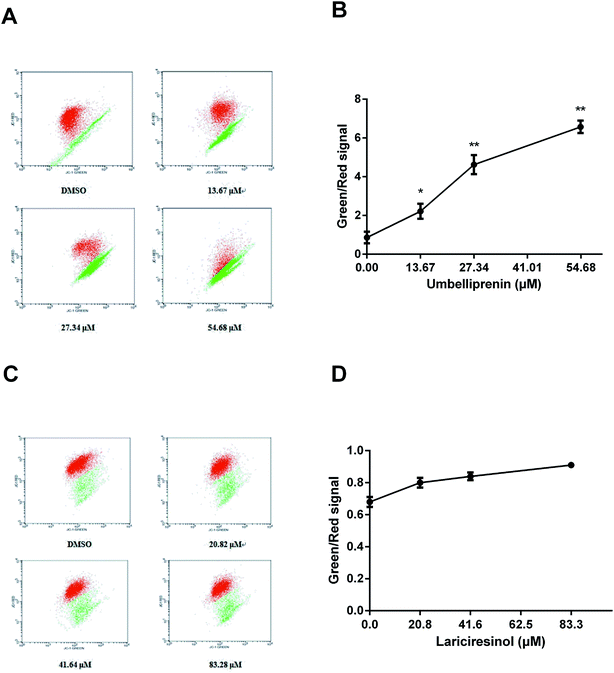
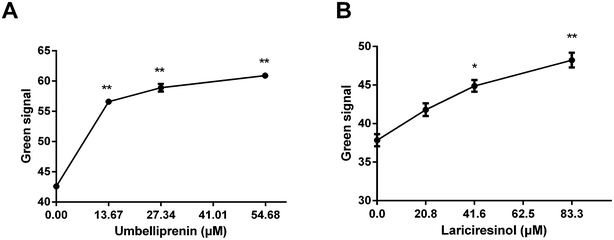
Apoptosis is also marked by the decrease of Δψ and JC-1 is often used as an indicator to detect Δψ during apoptosis. The decrease of MMP was measured as the increasing ratio of green-to-red fluorescence. As shown in Fig. 4, JC-1 fluorescence mostly appeared in red in the control group which indicated that the majority of cells were alive. UM treatment induced a significant increase of green fluorescence which indicated the loss of Δψ. The fluorescence ratios after UM treatment were higher than LA treatment, which indicated that apoptosis induction is associated with the loss of Δψ, with UM is more effective than LA.UM and LA affect the generation of ROS
ROS production was evaluated by the level of hydrogen peroxide production, using DCFH-DA detected by flow cytometry. After AGS cells were treated with UM or LA for 24 h, ROS production, as indicated by fluorescence, increased in a dose-dependent manner (Fig. 5). UM treatment (13.67 μM) caused a remarkable increase of fluorescence compared with control group; while the results of LA treatment showed slower increase of fluorescence respectively. Together, these data showed that both UM and LA could generate ROS in AGS cells, with UM being more effective.Effects of UM and LA on apoptosis-related protein expression in AGS gastric cancer cells
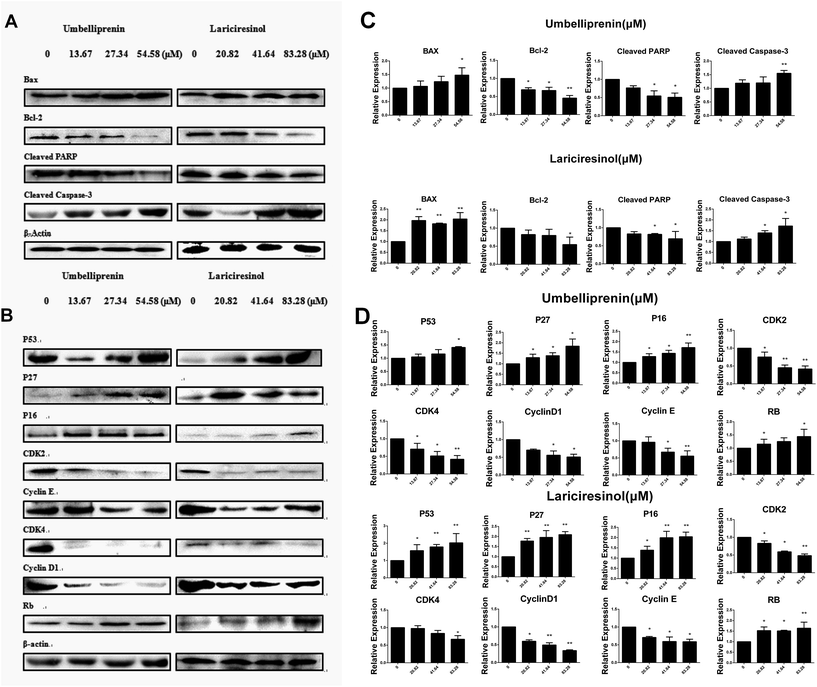
The Bcl-2 protein family is the key regulator of apoptosis.13 Our results showed that UM and LA both induced apoptosis in AGS cells. We then analyzed the protein expression of Bax and Bcl-2 after treating with UM or LA. Western blot results showed an increase in the level of Bax and a reduction of Bcl-2 protein after treatment with UM or LA (Fig. 6a). Caspase-3 is an executioner which cleaves a broad spectrum of cellular target proteins like nuclear PARP, leading to a cell death cascade. We examined the activation of caspase-3 and cleaved PARP after exposure to UM or LA. The results showed a decrease in cleaved PARP and an increase in cleaved caspase-3 (Fig. 6a). Relative protein expression results are shown in Fig. 6c. Combined with the JC-1 test, these results indicated that the mitochondrial apoptotic pathway is activated by UM and LA.UM and LA increase G0/G1 arrest of cell cycle in AGS cells
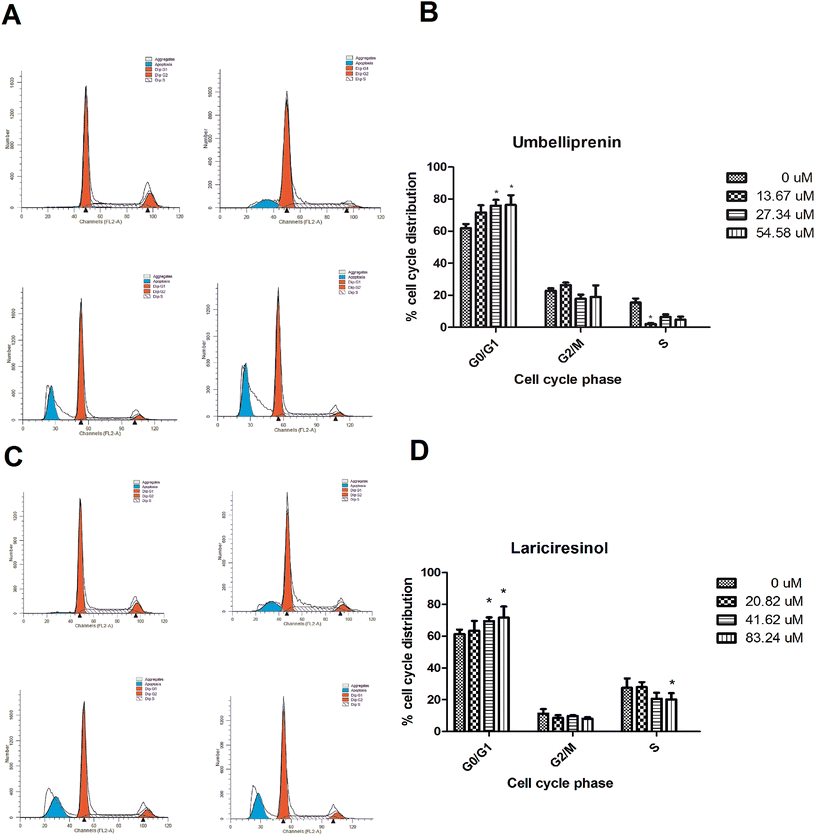
As UM and LA showed significant growth inhibition and effective apoptosis induction in AGS cells, we investigated their effects on cell cycle. AGS were treated with UM or LA for 24 h, followed by flow cytometry analyses. The UM-treated group showed G0/G1 phase arrest compared with control groups (Fig. 7a). Similar results were obtained when cells were treated with LA, with a slightly lower number of cells arrested in G0/G1 phase compared with the UM groups (76.26 ± 2.06% for UM and 71.61 ± 3.12% for LA) (Fig. 7b). The distribution of AGS cells in cell cycle treated by UM or LA are shown in Fig. 7c and d, indicating G0/G1 arrest in cell cycle. These results suggested that growth inhibition and apoptosis induction of UM and LA in AGS gastric cancer cells is at least partly associated with the induction of G0/G1 arrest in cell cycle.Effects of UM and LA on cell cycle regulation-related protein expression in AGS cells
To gain a further understanding about the molecular mechanisms in AGS cells during cell cycle arrest, we analyzed the effects of UM and LA on the expression of some major regulatory proteins. Fig. 6b showed that after treatment with UM or LA, the expression of cyclin D1, cyclin E, Cdk2 and Cdk4 decreased, while the expression of P27, P16 and Rb increased. Relative protein expression results are shown in Fig. 6d. These results suggest that changes of protein expression may play important roles in G0/G1 arrest of cell cycle in AGS cells.UM and LA inhibit tumor growth in BGC-823 tumor xenograft models
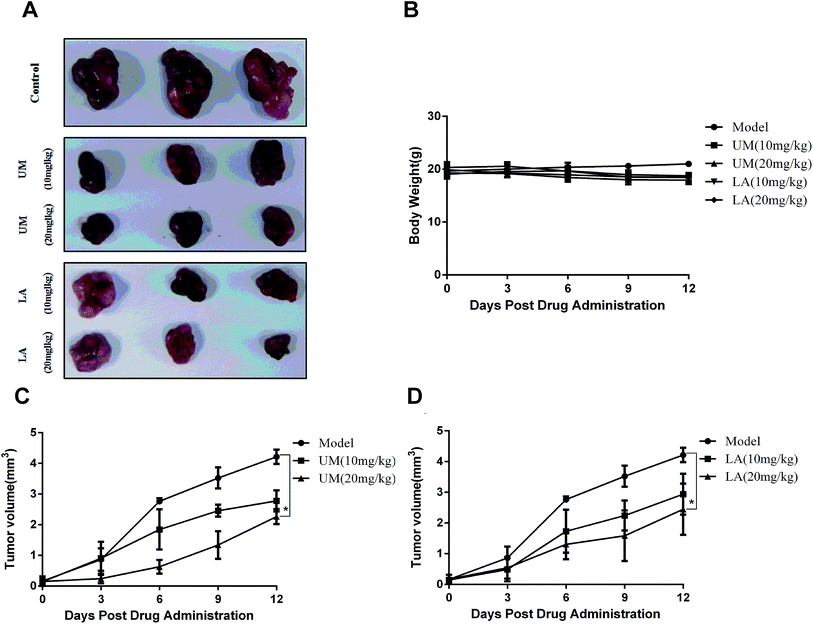
To assess the antitumor effects of UM and LA in xenograft models, human gastric cancer cells BGC-823 were inoculated subcutaneously into nude mice. Five groups of xenograft mice were administered with vehicle (control), 10 mg kg−1 and 20 mg kg−1 of UM, 10 mg kg−1 and 20 mg kg−1 of LA twice a day for 12 days and sacrificed at the end. The data showed that tumors from both UM and LA treatment groups grew slowly than the control group (Fig. 8c and d). In detail, no significant difference of the tumor volume in each group was observed at the beginning of treatment. At the end of treatment, tumor inhibition rates of UM were 63.64% (20 mg kg−1) or 40.81% (10 mg kg−1) and 43.33% (20 mg kg−1) or 37.24% (10 mg kg−1) in LA treatment groups when compared to the control group (Fig. 8a). The body weights in treatment groups slightly decreased during the treatment (Fig. 8b). Together, these data suggest that UM and LA effectively inhibit tumor growth.Discussion
Gastric cancer is one of the most common malignant diseases, and ranks second in mortality among all cancers worldwide.14 Currently, chemotherapy is used as the primary treatment for this disorder. However, overall survival rates of patients are low, while the incidences of side effects are high.15 Therefore, natural products with the potential for gastric cancer treatment have gained a lot of attention. F. sinkiangensis (called A-WEI in Mandarin) has been used as an effective medicine in treating stomach disorders in Xinjiang District of China for thousands of years.16 In addition, there have been recent studies on the isolation of compounds from the roots and volatile oil from F. sinkiangensis.10,17 However, studies on the efficacy and mechanism of the anti-gastric cancer effects of this valuable medicine have not been reported yet. Our previous results showed that a petroleum ether extract from the seeds exhibit antitumor effect in vivo. Based on these observations we performed a further research on the compounds isolated from the seeds. In the present study, we found seven compounds that show anti-gastric cancer activity. After screening, we found UM and LA were the most effective compounds in inhibiting the growth of AGS gastric cancer cells among the seven compounds tested. We then took these two compounds as potential therapeutic agents in gastric cancer treatment for further study.In this study, UM and LA showed the best antitumor activity against AGS cells with lower toxicity against normal human gastric epithelial cells and other cancer cell lines, suggesting that UM and LA could be both effective and specific agents against human gastric cancer cells. Cell selectivity related with multiple factors such as metabolism and interaction with specific receptors and tumor microenvironment. There have been researches on the relations between compounds and biological targets in their selectivity for cancer cells. For example, a series of 6,7,10-trimethoxy-α-naphthoflavones (4a–o) were synthesized and their inhibitory potency against cancer cells related with their selectivity for CYP1B1 in human breast adenocarcinoma MCF-7 cell line.18 And purine-scaffold compound series showed cell selectivity for different effects on Grp94 which could regulate intracellular trafficking of Toll-like receptor 9.19 There has also been research on tumor microenvironment showed that the inhibitory effect of iron chelator DIBI on human and murine mammary carcinoma and fibrosarcoma cells varies for the change of tumor microenvironment.20 Besides these factors the inhibition of cancer cell proliferation is often associated with cell cycle arrest.21 The G1 phase is a key part of the cell cycle. It is involved in the pathogenesis of many diseases, and also the entry point for many drug therapies.22 The few reports on the effects of UM on cell cycle progression demonstrate that UM arrests the growth of human M4Beu metastatic pigmented malignant melanoma cells by G1 arrest,23 whereas no reports on the effects of LA on cell cycle have been reported. Our results showed that UM and LA arrest AGS cells in G0/G1 phase, which prevents the conversion to the S phase and M phase, suggesting one possible mechanism for cell cycle arrest by UM and LA. Western blot results showed that cyclin D, cyclin E, Cdk4 and Cdk2 were down-regulated after treatments, while Cdk inhibitors P27, P16, and downstream Rb were up-regulated. The Cdk inhibitors have been shown to arrest the cell cycle and inhibit the growth of cancer cells.24 These results may therefore provide an additional explanation for the G0/G1 phase arrest induced by UM and LA.
Apoptosis induction plays an important role in the inhibition of tumor cells.25 Although UM and LA have been shown to induce apoptosis in some cancer cells,23,26–28 the effects on anti-gastric cancer cells have not been reported, and the mechanism is not fully understood. In the present study, after AO/EB and Hoechst 33342 staining, AV/PI apoptosis detection, and changes in MMP and ROS tests, we found that both UM and LA can induce apoptosis in gastric cancer AGS cells. When the cells were active in the apoptotic pathway, the signal caused the activation of caspase-3, which led to apoptosis.29,30 Western blot analysis showed that both UM and LA can activate caspase-3, cleaving the substrate PARP at the same time. We also found an increase in pro-apoptotic protein Bax expression and a decrease in anti-apoptotic protein Bcl-2 expression in AGS cells. An increase in the ratio of Bax/Bcl-2 stimulates the induction of apoptosis.31 These results support the roles of caspase-3 and Bcl-2 family proteins in both UM and LA-induced apoptosis in gastric cancer cells. The in vivo anticancer activity of UM and LA were evaluated in human gastric cancer BGC-823 xenograft models. The results showed that both UM and LA can reduce the volume of tumor in vivo.
Notably, although the IC50 values of UM and LA in cells varies, the protein expression levels after treatment by the two compounds showed no obvious differences. The effects of LA lag behind UM and could be a contributing factor to these results. Thus, the mechanisms involving the relationships between structure and function are not clear, and further investigations are necessary.
In conclusion, we found UM and LA were the most specific and effective compounds in the growth inhibition of human gastric cancer AGS cells among the seven compounds which were first isolated from the seed of F. sinkiangensis K. M. Shen. UM and LA could induce apoptosis in AGS cells with increased Bax/Bcl-2 ratios, the generation of ROS, and the decrease of MMP. UM and LA could also induce G0/G1 phase arrest in cell cycle through the regulation of the G0/G1 phase checkpoint proteins. Therefore, UM and LA could be treated as valuable candidates for further investigation as possible antitumor treatments for gastric cancer.
Contributions
L.C. and X.S. designed the experiments; X.L. identified the plant material; J.Y. and G.Z. isolated compounds. Li.Z., Le.Z., L.G., X.H. and D.L. performed the experiments; Li.Z. analyzed the data and wrote the manuscript. All authors reviewed the manuscript and approved it for submission.Conflict of interest
The authors declare no competing financial interests.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81460661) and the Program for Innovative Research Team in IMPLAD (PIRTI). This work was also supported by the Ministry of Science and Technology of the People's Republic of China, and Major Scientific and Technological Special Project for “Significant New Drugs Formulation” (Grant No. 2012ZX09501001-004 and 2012ZX09301-002-001-026).References
- Y. J. Yu, W. J. Sun, M. D. Lu, F. H. Wang, D. S. Qi, Y. Zhang, P. H. Li, H. Huang, T. You and Z. Q. Zheng, World J. Gastroenterol., 2014, 20, 18413–18419 CrossRef PubMed.
- R. Siegel, J. Ma, Z. Zou and A. Jemal, Ca-Cancer J. Clin., 2014, 64, 9–29 CrossRef PubMed.
- C. Pinto, F. Fabio, S. Siena, S. Casxinu, L. F. L. Rojas, C. Ceccarelli, V. Mutri, L. Giannetta, S. Giaquinta, C. Funaioli, R. Berardi, C. Longobardi, E. Piana and A. A. Martoni, Ann. Oncol., 2007, 2007(18), 510–517 Search PubMed.
- V. E. Cutsem, Proc ASCO., Abstract, LBA4509, 2009 Search PubMed.
- F. H. Cortés, F. Rivera, I. Alés, A. Márquez, A. Velasco, R. Colomer, C. R. Garcia, J. Sastre, J. Guerra and C. Gravalos, Ann Oncol., 2007, 25, 4613 Search PubMed.
- R. Qin, H. Shen, Y. Cao, Y. Fang, H. Li, Q. Chen and W. Xu, PLoS One, 2013, 8, e76486 CAS.
- M. Y. Xu, D. H. Lee, E. J. Joo, K. H. Son and Y. S. Kim, Food Chem. Toxicol., 2013, 59, 703–708 CrossRef CAS PubMed.
- S. He and D. Y. Tan, J Xinjiang Agr Univ., 2005, 25, 1–7 Search PubMed.
- H. Zhang and J. Hu, Chin Pharmacol Bull., 1987, 3, 288–290 Search PubMed.
- J. R. Yang, Z. An, Z. H. Li, S. Jing and H. L. Qina, Chem Pharm bull., 2006, 54, 1595–1598 CrossRef CAS.
- G. Z. Li, X. J. Li, L. Cao, L. G. Shen, J. Zhu, J. Zhang, J. C. Wang, L. J. Zhang and J. Y. Si, Fitoterapia, 2014, 97, 247–252 CrossRef CAS PubMed.
- Y. E. Wang, J. Y. Si, X. J. Li, L. Jiang and J. Zhu, Mod Chin Med., 2011, 13, 26–28 CAS.
- W. A. Siddiqui, A. Ahad and H. Ahsan, Arch. Toxicol., 2015, 89, 289–317 CrossRef CAS PubMed.
- M. B. Piazuelo and P. Correa, Colombia Médica, 2013, 44, 192–201 Search PubMed.
- X. Paoletti, K. Oba, T. Burzykowski, S. Michiels, Y. Ohashi, J. P. Pignon, P. Rougier, J. Sakamoto, D. Sargent, M. Sasako, E. van Cutsem and M. Buyse, Jama, 2010, 303, 1729–1737 CrossRef CAS PubMed.
- C. Tian, L. Q. Xie and G. L. Li, Seed, 2008, 5, 88–90 Search PubMed.
- B. Ye, S. Wang and L. Zhang, Nat. Prod. Res., 2011, 25, 1161–1170 CrossRef CAS PubMed.
- J. H. Zhang, Q. Q. Meng, X. Zhang, Q. Cui, W. Zhou and S. S. Li, J. Med. Chem., 2015, 58, 3534–3547 CrossRef PubMed.
- H. J. Patel, P. D. Patel, S. O. Ochiana, P. R. Yan, W. L. Sun, M. R. Patel, S. K. Shah, E. Tramentozzi, J. Brooks, A. Bolaender, L. Shrestha, R. Stephani, P. Finotti, C. Leifer, Z. H. Li, D. T. Gewirth, T. Taldone and G. Chiosis, J. Med. Chem., 2015, 58, 3922–3943 CrossRef CAS PubMed.
- M. R. P. Coombs, T. Grant, A. L. Greenshields, D. J. Arsenault, B. E. Holbein and D. W. Hoskin, Exp. Mol. Pathol., 2015, 99, 262–270 CrossRef PubMed.
- R. Marcotte and E. Wang, J. Gerontol., 2002, 57, B257–B269 CrossRef PubMed.
- M. Narita, S. Nunez, E. Heard, M. Narita, A. W. Lin, S. A. Hearn, D. L. Spector, G. J. Hannon and S. W. Lowe, Cell, 2003, 113, 703–716 CrossRef CAS.
- C. Barthomeuf, S. Lim, M. Iranshahi and P. Chollet, Phytomedicine, 2008, 15, 103–111 CrossRef CAS PubMed.
- M. Malumbres and M. Barbacid, Nat. Rev. Cancer, 2009, 9, 153–166 CrossRef CAS PubMed.
- S. W. Lowe and A. W. Lin, Carcinogenesis, 2000, 21, 485–495 CrossRef CAS PubMed.
- N. Khaghanzadeh, Z. Mojtahedi, M. Ramezani, N. Erfani and A. Ghaderi, Daru, 2012, 20, 69–74 CrossRef CAS PubMed.
- S. A. Ziai, O. Gholami, M. Iranshahi, A. H. Zamani and M. Jeddi-Tehrani, Iran. J. Pharm. Res., 2012, 11, 653–659 CAS.
- F. Bakar, M. G. Caglayan, F. Onur, S. Nebioglu and I. M. Palabiyik, Anti-Cancer Agents Med. Chem., 2014, 15, 336–344 CrossRef.
- N. N. Danial, Clin. Cancer Res., 2007, 13, 7254–7263 CrossRef CAS PubMed.
- T. T. Huang, S. P. Wu, K. Y. Chong, D. M. Ojcius, Y. F. Ko, Y. H. Wu, C. Y. Wu, C. C. Lu, J. Martel, J. D. Young and H. C. Lai, J. Ethnopharmacol., 2014, 155, 154–164 CrossRef PubMed.
- J. Han, P. Tan, Z. Li, Y. Wu, C. Li, Y. Wang, B. Wang, S. Zhao and Y. Liu, PLoS One, 2014, 9, e86539 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11335k |
| This journal is © The Royal Society of Chemistry 2015 |