Emulsion polymerization for the fabrication of poly(o-phenylenediamine)@multi-walled carbon nanotubes nanocomposites: characterization and their application in the corrosion protection of 316L SS†
Abstract
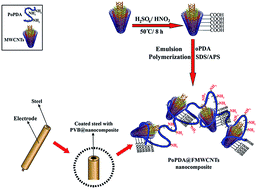
Recently, the corrosion of metals is an important academic and industrial concern which has received significant attention. Poly(o-phenylenediamine) (PoPDA) based nanocomposites with unfunctionalized multi-walled carbon nanotubes (MWCNTs) and functionalized multi-walled carbon nanotubes (FMWCNTs) were prepared through emulsion polymerization using sodium dodecyl sulfate (SDS) as an emulsifier and ammonium persulfate (APS) as an oxidant. Fourier transform infrared (FT-IR) spectra confirmed the construction of the nanocomposites. The morphology of the synthesized nanocomposites was characterized using scanning electron microscopy (SEM). X-ray diffraction (XRD) patterns of the nanocomposites indicated a more crystalline nature than that of bare PoPDA. The thermal stability of the nanocomposites was improved relative to bare PoPDA. The corrosion protection performance of the coatings containing PoPDA@MWCNTs, PoPDA@FMWCNTs and PoPDA on steel was evaluated using potentiodynamic polarization, electrochemical impedance spectroscopic (EIS) and open circuit potential (OCP) measurements in 3.5% NaCl solution. The obtained results of the potentiodynamic polarization, EIS and OCP showed that the PoPDA@MWCNT nanocomposite coated steel had excellent corrosion inhibition behavior in saline solution.

 Please wait while we load your content...
Please wait while we load your content...