Analysis of the catalytic activity induction and deactivation of PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction
Ke Xia,
Wan-Zhong Lang*,
Pei-Pei Li,
Xi Yan and
Ya-Jun Guo*
The Education Ministry Key Laboratory of Resource Chemistry and Shanghai Key Laboratory of Rare Earth Functional Materials, Department of Chemistry and Chemical Engineering, Shanghai Normal University, 100 Guilin Road, Shanghai 200234, China. E-mail: wzlang@shnu.edu.cn; guoyajun@shnu.edu.cn; Fax: +86-21-64321951; Tel: +86-21-64321951
First published on 14th July 2015
Abstract
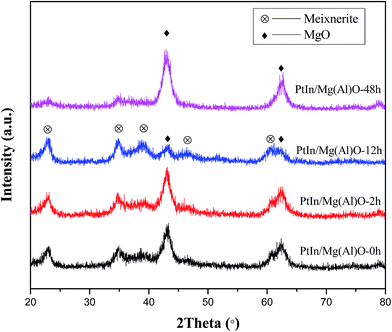
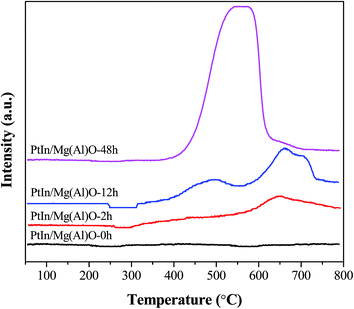
The catalytic activity induction and deactivation of PtIn/Mg(Al)O catalysts for propane dehydrogenation reaction are experimentally verified. Numerous physical–chemical characterizations are employed to probe the basis and structure–activity relationships, and a mechanism for the activity induction and deactivation is proposed with the help of a schematic diagram. XPS results prove that the valence state of In exhibits almost no change during the entire dehydrogenation reaction. In the activity induction period, the average metal particle size of the PtIn/Mg(Al)O catalyst presents a decreasing trend, and the specific surface area increases. Moreover, the crystal phase changes from primarily periclase (MgO) to dominantly meixnerite (Mg6Al2(OH)18·4H2O). Coke is mainly deposited on the carrier. Nevertheless, in the deactivation period, the metal particles tend to agglomerate and grow. The specific surface area decreases and crystal phase returns to the unique periclase crystal phase. A large amount of coke is formed over the catalyst and partially covers the active sites, which leads to the evident decrease of catalytic activity.
1 Introduction
In recent years, the conversion of low alkanes to value-added olefins has drawn great attention, because of the fact that light alkenes are extensively utilized as raw materials in the polymer industry and for synthesis of other commercial chemicals.1–4 Currently, studies on propane dehydrogenation (PDH) are mainly focused on the preparation of high-efficiency catalysts to attain a desirable yield of propylene.5It is well known that the deactivation of catalysts for alkane dehydrogenation is inevitable due to the formation of coke and the agglomeration of active metallic particles.6 However, increase in the catalytic activity rather than the deactivation phenomenon was observed in the initial stage of the propane dehydrogenation reaction over In2O3/MOx (M = Al, Zr) catalysts by Chen et al.7–9 They verified that this unique phenomenon could be associated with the induction period of developing the active sites attributed to the in situ creation of In0 species, which is confirmed as the intrinsic active center for dehydrogenation. A similar induction period has also been reported for propane dehydrogenation over an Fe2O3/Al2O3 catalyst,10 which was ascribed to the variation of the valence state of Fe species. Moreover, it was proposed that MoAl11 and Mo/MgAl2O4 (ref. 12 and 13) catalysts exhibited similar activity induction for butane dehydrogenation, which was due to the in situ formation of active sites from well-dispersed Mo species under the reaction conditions.
Recently, calcined hydrotalcite (referred to as Mg(Al)O) was proposed as an efficient support for Pt-based catalysts in propane dehydrogenation reaction.14,15 This types of materials possess moderate basic properties and high thermal stability. It was reported that Pt/Mg(Al)O and PtSn/Mg(Al)O catalysts were investigated by Galvita et al.14 with the aim of understanding the effects of Sn and the formation of coke for ethane dehydrogenation. Akporiaye et al.16 reported a series of PtSn/Mg(Al)O catalysts to optimize the formulation procedure and identify the preparation conditions that result in the best catalytic performance for propane dehydrogenation reaction. Subsequently, Siddiqi et al.17 revealed the performance of Pt/Mg(Ga)(Al)O catalysts for the dehydrogenation of ethane and propane, and they compared the activity, stability, and coking characteristics of Pt/Mg(Al)O, PtGa/Mg(Al)O and PtSn/Mg(Al)O catalysts. Furthermore, Sun et al.18 and Wu et al.19 reported novel Pt/Mg(In)(Al)O catalysts to examine the effect of reduction temperature on PtIn alloy formation and investigated the effects of In/Pt ratio on the intrinsic performances and coking of Pt/Mg(In)(Al)O. In all the reports, it is notable that the investigations were devoted to optimal catalytic performance by probing structure–performance relationships, and no activity induction took place over the calcined hydrotalcite (or hydrotalcite-like) supported Pt-based catalysts during the entire dehydrogenation reaction. Nevertheless, an interesting activity induction period was found over the PtIn/Mg(Al)O catalyst for propane dehydrogenation in this study.
The objective of this study was to analyze the variation of the catalytic activity of PtIn/Mg(Al)O catalyst for propane dehydrogenation. The Mg(Al)O support was first synthesized, and then Pt and In were deposited onto the support to obtain a PtIn/Mg(Al)O catalyst using the successive impregnation method. As a reference, PtIn/Al and PtIn/Mg catalysts were also prepared similarly and their catalytic performances were evaluated in propane dehydrogenation reaction. Several analytical techniques, including XRD, BET, TEM, XPS and TPO, were carried out to explore the reasons for the variation of catalytic activity.
2 Experimental
2.1 Preparation of catalysts
Calcined hydrotalcite (referred as Mg(Al)O) was prepared by co-precipitation and subsequent heat treatment.14 Mg(NO3)2·6H2O and Al(NO3)3·9H2O (depending on the desired Mg/Al ratio, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were dissolved in deionized water. Another solution was prepared with certain amounts of Na2CO3 and NaOH dissolved in deionized water. Then, the two solutions were slowly added dropwise to a glass flask with stirring at 60 °C for 20 min. The solution pH was maintained at approximately 9–10. Subsequently, the mixed solution was aged at room temperature for 18 h. The precipitate was filtered, washed with distilled water to neutrality, and dried in air at 110 °C for 10 h. The dried hydrotalcite (HT) was calcined at 700 °C for 10 h to obtain the calcined support (Mg(Al)O).
1) were dissolved in deionized water. Another solution was prepared with certain amounts of Na2CO3 and NaOH dissolved in deionized water. Then, the two solutions were slowly added dropwise to a glass flask with stirring at 60 °C for 20 min. The solution pH was maintained at approximately 9–10. Subsequently, the mixed solution was aged at room temperature for 18 h. The precipitate was filtered, washed with distilled water to neutrality, and dried in air at 110 °C for 10 h. The dried hydrotalcite (HT) was calcined at 700 °C for 10 h to obtain the calcined support (Mg(Al)O).
PtIn/Mg(Al)O catalysts were prepared using the sequential impregnation method. The calcined support, Mg(Al)O, was firstly impregnated with an InNO3 aqueous solution, followed by an H2PtCl6 solution. After each impregnation step, the samples were dried at 50 °C for 3 h, then dried at 120 °C for 2 h, and finally calcined at 550 °C for 4 h. The contents of Pt and In in the catalysts were fixed at 0.6 wt% and 1.5 wt%, respectively. For reference, PtIn/Al and PtIn/Mg catalysts were also prepared similarly using commercial γ-Al2O3 (SBET = 222.0 m2 g−1) and MgO (SBET = 111.4 m2 g−1) in powdered form as the support, respectively. The spent PtIn/Mg(Al)O catalysts undergoing reaction for different times were denoted as PtIn/Mg(Al)O-M (M = 0, 2, 12 and 48 h, where M represents reaction time). All the raw materials were purchased from Sinopharm Chemical Reagent Co., Ltd (China).
2.2 Characterization of catalysts
X-ray diffraction (XRD) patterns of PtIn/Mg(Al)O-M catalysts were recorded on a Bragg–Brentano diffractometer (Rigaku D/Max-2000) using monochromatic Cu Kα radiation (λ = 1.5418 Å). The samples were scanned from the 2θ value of 20° to 80° with a scan speed of 4° min−1. The X-ray tube was operated at 40 kV and 30 mA.The textural properties of PtIn/Mg(Al)O-M catalysts were analyzed by N2 adsorption–desorption at liquid nitrogen temperature using an automatic analyzer (NOVA 4000, Quantachrome, USA). The samples were outgassed for 4 h under vacuum at 300 °C prior to adsorption. Specific surface areas of the samples were calculated using the Brunauer–Emmett–Teller (BET) method. The Barrett–Joyner–Halenda (BJH) pore size model was used to calculate the average pore diameter from the adsorption branch of the isotherm.
Transmission electron microscopy (TEM) images were obtained using a JEM-2010 microscope operated at 200 kV. Reduced samples were prepared by dispersing and sonicating the catalysts in ethanol, then placing a small drop of this solution onto carbon-film coated copper grids and drying in air before testing. Approximately one hundred individual metal particles were analyzed to determine the average particle size.
X-ray photoelectron spectra (XPS) of PtIn/Mg(Al)O-M catalysts were obtained on a Perkin-Elmer PHI 5000C ESCA using Al Kα radiation. All the samples were pre-reduced in situ under hydrogen at 580 °C for 2.5 h. Binding energies (BE) were calibrated using the C1s level at 284.8 eV as an internal standard.
Temperature-programmed oxidation (TPO) experiments were conducted in a programmable temperature system. Prior to TPO analysis, the spent catalysts (0.05 g) were purged in flowing N2 (15 mL min−1) at 500 °C for 1 h. Then, the temperature was lowered to 40 °C to steady the baseline under this gas flow. Subsequently, the reactor was heated in a mixture of 10% O2 in He from room temperature (RT) to 800 °C at 10 °C min−1. Finally, a thermal conductivity detector (TCD) cell was used to detect CO2.
2.3 Catalytic activity measurements
Propane dehydrogenation reactions were performed in a conventional fix-bed quartz reactor. The catalyst (0.3 g) was placed in the quartz reactor and pre-reduced under H2 at 580 °C for 2.5 h before evaluation. The reaction conditions were as follows: the reaction temperature was 620 °C, pressure was 0.1 MPa, molar ratio of H2/C3H8/Ar was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, and the propane weight hourly space velocity (WHSV) was 3.3 h−1. A gas chromatography apparatus (GC, SP-6890) equipped with an FID detector and an AT-PLOT PORA-Q capillary column was employed to analyze the outlet gas mixtures. Propane conversion and propylene selectivity were defined as follows:
35, and the propane weight hourly space velocity (WHSV) was 3.3 h−1. A gas chromatography apparatus (GC, SP-6890) equipped with an FID detector and an AT-PLOT PORA-Q capillary column was employed to analyze the outlet gas mixtures. Propane conversion and propylene selectivity were defined as follows:| C3H8 conversion = (C3H8in − C3H8out)/C3H8in | (1) |
| C3H6 selectivity = C3H6out/(C3H8in − C3H8out) | (2) |
3 Results and discussion
3.1 The activity induction and deactivation phenomena of the PtIn/Mg(Al)O catalyst for propane dehydrogenation
Fig. 1 displays the catalytic performances of different catalysts. Propane conversions with the time on stream for different catalysts are presented in Fig. 1(a). It can be seen that the PtIn/Al catalyst exhibits the highest initial activity among the three catalysts, but its activity decreases rapidly with increasing time on stream. A low, but stable activity (around 11.0% propane conversion) can be obtained over the PtIn/Mg catalyst. Moreover, as shown in Fig. 1(b), the PtIn/Al and PtIn/Mg(Al)O catalysts possess a high selectivity for propylene (about 96.0%). In comparison, a relatively low selectivity of around 70.0% can be noted over the PtIn/Mg sample.From Fig. 1(a), it is interesting to note that the catalytic activity of the PtIn/Mg(Al)O catalyst increases continually during the entire 155 min reaction, which is inconsistent with the inevitable deactivation of the catalysts for propane dehydrogenation. However, it is noteworthy that this phenomenon cannot be observed over the other two catalysts. On the basis of the aforementioned results, it can be concluded that it is peculiar for the Mg(Al)O material-supported PtIn bimetallic catalyst to present constantly increasing activity, which is called as the ‘activity induction period’.
To further probe the variation of the catalytic activity, a longer reaction time (50 h) for consecutive propane dehydrogenation at 620 °C was investigated over the PtIn/Mg(Al)O catalyst. As can be seen from Fig. 2, the variation of propane conversion can be divided into three stages: (1) ascent stage (0–8 h), (2) stable stage (8–24 h) and (3) descent stage (24–50 h). The initial propane conversion (5 min of reaction time) is 46.1%, which rises constantly until 8 h. Then, it remains stable at around 61.0% for 16 h. Subsequently, the conversion begins to decline gradually, and the final value is 42.0% after 50 h of propane dehydrogenation reaction. The propylene selectivity varies slightly (about 95%) during the entire dehydrogenation reaction process. The spent PtIn/Mg(Al)O catalysts undergoing different reaction times (denoted as PtIn/Mg(Al)O-M, M = 0, 2, 12 and 48 h represents reaction time) were cooled and used for the following structure analysis and characterizations.
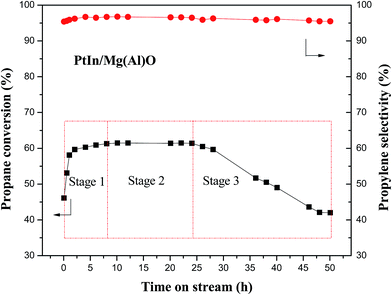 | ||
Fig. 2 Stability test of the PtIn/Mg(Al)O catalyst in propane dehydrogenation reaction (reaction conditions: 620 °C, H2/C3H8/Ar (molar ratio) = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, WHSV = 3.3 h−1, m(cat) = 0.3 g). 35, WHSV = 3.3 h−1, m(cat) = 0.3 g). | ||
3.2 Analysis of the activity induction and deactivation of the PtIn/Mg(Al)O catalyst
 | ||
| Fig. 5 TEM micrographs and particle size distribution histograms of the PtIn/Mg(Al)O-M catalysts: (a) PtIn/Mg(Al)O-0h, (b) PtIn/Mg(Al)O-2h, (c) PtIn/Mg(Al)O-12h and (d) PtIn/Mg(Al)O-48h. | ||
| Sample name | SBET (m2 g−1) | Vp (cm3 g−1) | Dp (nm) | Average Pt particle size (nm) |
|---|---|---|---|---|
| PtIn/Mg(Al)O-0h | 182.9 | 0.2201 | 2.818 | 2.5 |
| PtIn/Mg(Al)O-2h | 199.3 | 0.1798 | 1.974 | 2.3 |
| PtIn/Mg(Al)O-12h | 279.4 | 0.2081 | 1.870 | 1.3 |
| PtIn/Mg(Al)O-48h | 174.4 | 0.2023 | 2.157 | 5.3 |
3.3 The mechanism of activity induction and deactivation of the PtIn/Mg(Al)O catalyst
According to coke deposition and Pt particle size of the PtIn/Mg(Al)O catalyst as a function of time on stream, a schematic diagram of the mechanism is proposed in Fig. 7. In the abovementioned XPS analysis, the enrichment of In2O3 on the surface of the PtIn/Mg(Al)O catalyst is verified. On the other hand, In promotes the transfer of coke from the active sites to the support (in TPO analysis), which is similar to the effect of Sn. From the structure of the Pt–SnOx support proposed in the literature,27 it can be inferred that Pt metal particles are highly dispersed on the support, which is attributed to the similar “sandwich structure” of the Pt–In2O3-support. In this case, the average Pt particle size is about 2.5 nm. When the reaction time increases to 2 h, coke deposition begins to occur on the support, and very little coke is deposited on the metallic active sites, thus no deactivation of the PtIn/Mg(Al)O catalyst can be found in the initial stage (stage 1). The smallest size of Pt particles over the catalyst can be obtained after 12 h of reaction. Here, the main crystalline phase of the carrier changes and results in the formation of a higher specific surface area, which gives rise to the variation of Pt particles size. Furthermore, as the reaction progresses, the average Pt particles agglomerate due to the prolonged high temperature reaction. Thus, the average Pt particle size of 5.3 nm is observed for PtIn/Mg(Al)O-48h. Besides, a large amount of coke is formed over the catalyst, which partially covers the active sites; this leads to an evident decrease in the catalyst activity.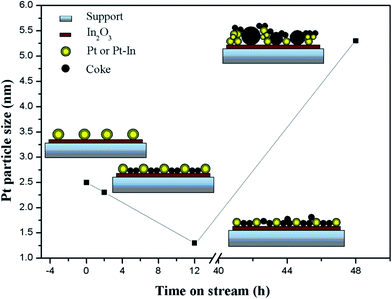 | ||
| Fig. 7 Schematic of carbon deposition and Pt particle size of the prereduced PtIn/Mg(Al)O catalyst as a function of time on stream. | ||
4 Conclusions
In this study, Mg(Al)O materials were synthesized by the coprecipitation method and used as supports for bimetallic PtIn catalysts for propane dehydrogenation to propylene. A peculiar phenomenon of increasing activity in the initial stage could be observed over the PtIn/Mg(Al)O catalyst. The catalytic activity induction and deactivation of the PtIn/Mg(Al)O catalyst for the propane dehydrogenation reaction were experimentally verified. XPS results prove that the valence state of In exhibits almost no change during the entire dehydrogenation reaction. As the reaction proceeds, smaller metal particle size, better distribution of Pt particles, higher specific surface area and dominant crystalline phase of meixnerite (Mg6Al2(OH)18·4H2O) can be obtained over the PtIn/Mg(Al)O catalyst. TPO curves verify that carbon is deposited on the support before it gets deposited over the active sites. However, the agglomeration of metal particles and large amount of coke formed covers the active sites, which give rise to marked decline in activity after 48 h of reaction.The catalytic activity of the PtIn/Mg(Al)O catalyst goes through ascent, stable and descent stages. The activity variation is attributed to the changes in the metal particle size, the state of coke deposition, the specific surface area and the crystalline phase of the catalyst.
Acknowledgements
The research is supported by Science and Technology Commission of Shanghai Municipality (13ZR1429900, 14520502900) and Internation Joint Laboratory on Resource Chemistry (IJLRC).References
- R. Grabowski, Catal. Rev., 2006, 48, 199–268 CAS.
- P. P. Sun, G. Siddiqi, M. F. Chi and A. T. Bell, J. Catal., 2010, 274, 192–199 CrossRef CAS PubMed.
- D. Shee and A. Sayari, Appl. Catal., A, 2010, 389, 155–164 CrossRef CAS PubMed.
- S. Sahebdelfar, P. M. Bijani, M. Saeedizad, F. T. Zangeneh and K. Ganji, Appl. Catal., A, 2011, 395, 107–113 CrossRef CAS PubMed.
- Y. B. Xu, J. Y. Lu, M. Zhong and J. D. Wang, Progr. Chem., 2008, 20, 650–656 CAS.
- Z. Nawaz and F. Wei, J. Ind. Eng. Chem., 2010, 16, 774–784 CrossRef CAS PubMed.
- M. Chen, J. Xu, Y. Cao, H.-Y. He, K.-N. Fan and J.-H. Zhuang, J. Catal., 2010, 272, 101–108 CrossRef CAS PubMed.
- M. Chen, J.-L. Wu, Y.-M. Liu, Y. Cao, L. Guo, H.-Y. He and K.-N. Fan, Appl. Catal., A, 2011, 407, 20–28 CrossRef CAS PubMed.
- M. Chen, J. Xu, Y. M. Liu, Y. Cao, H. Y. He and J. H. Zhuang, Appl. Catal., A, 2010, 377, 35–41 CrossRef CAS PubMed.
- Y. Sun, Y. Wu, L. Tao, H. Shan, G. Wang and C. Li, J. Mol. Catal. A: Chem., 2015, 397, 120–126 CrossRef CAS PubMed.
- M. E. Harlin, L. B. Backman, A. O. I. Krause and O. J. T. Jylhä, J. Catal., 1999, 183, 300–313 CrossRef CAS.
- G. W. Wang, C. Y. Li, H. H. Shan and W. L. Wu, Ind. Eng. Chem. Res., 2013, 52, 13297–13304 CrossRef CAS.
- G. Wang, N. Sun, C. Gao, X. Zhu, Y. Sun, C. Li and H. Shan, Appl. Catal., A, 2014, 478, 71–80 CrossRef CAS PubMed.
- V. Galvita, G. Siddiqi, P. P. Sun and A. T. Bell, J. Catal., 2010, 271, 209–219 CrossRef CAS PubMed.
- E. A. Redekop, V. V. Galvita, H. Poelman, V. Bliznuk, C. Detavernier and G. B. Marin, ACS Catal., 2014, 4, 1812–1824 CrossRef CAS.
- D. Akporiaye, S. F. Jensen, U. Olsbye, F. Rohr, E. Rytter, M. Ronnekleiv and A. I. Spjelkavik, Ind. Eng. Chem. Res., 2001, 40, 4741–4748 CrossRef CAS.
- G. Siddiqi, P. P. Sun, V. Galvita and A. T. Bell, J. Catal., 2010, 274, 200–206 CrossRef CAS PubMed.
- P. P. Sun, G. Siddiqi, W. C. Vining, M. F. Chi and A. T. Bell, J. Catal., 2011, 282, 165–174 CrossRef CAS PubMed.
- J. Wu, Z. M. Peng, P. P. Sun and A. T. Bell, Appl. Catal., A, 2014, 470, 208–214 CrossRef CAS PubMed.
- X. Liu, W.-Z. Lang, L.-L. Long, C.-L. Hu, L.-F. Chu and Y.-J. Guo, Chem. Eng. J., 2014, 247, 183–192 CrossRef CAS PubMed.
- X. Q. Fan, J. M. Li, Z. Zhao, Y. C. Wei, J. Liu, A. J. Duan and G. Y. Jiang, Catal. Sci. Technol., 2015, 5, 339–350 CAS.
- P. C. H. Mitchell and S. A. Wass, Appl. Catal., A, 2002, 225, 153–165 CrossRef CAS.
- J. Perez-Ramirez, S. Abello and N. M. van der Pers, Chem.–Eur. J., 2007, 13, 870–878 CrossRef CAS PubMed.
- M. Mokhtar, A. Inayat, J. Ofili and W. Schwieger, Appl. Clay Sci., 2010, 50, 176–181 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhou, L. Wan, M. Xue, Y. Duan and X. Liu, Fuel Process. Technol., 2011, 92, 1632–1638 CrossRef CAS PubMed.
- Y. W. Zhang, Y. M. Zhou, J. J. Shi, S. J. Zhou, Z. W. Zhang, S. C. Zhang and M. P. Guo, Fuel Process. Technol., 2013, 111, 94–104 CrossRef CAS PubMed.
- Z. Nawaz, X. P. Tang, Y. Wang and F. Wei, Ind. Eng. Chem. Res., 2010, 49, 1274–1280 CrossRef CAS.
- Y. W. Zhang, Y. M. Zhou, L. H. Wan, M. W. Xue, Y. Z. Duan and X. Liu, J. Nat. Gas Chem., 2011, 20, 639–646 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |