A novel magnetic fluorescent chemosensor for Cu2+ based on self-assembled systems of azobenzene and α-cyclodextrin†
Yue Zhanga,
Qiang Lia,
Jing Guoa,
Yaoxian Lia,
Qingbiao Yang*a and
Jianshi Du*b
aDepartment of Chemistry, Jilin University, Changchun, 130021, P. R. China. E-mail: yangqb@jlu.edu.cn
bChina Japan Union Hospital, Jilin University, Changchun, 130031, P. R. China. E-mail: dujianshi3043@126.com
First published on 27th July 2015
Abstract
A novel fluorescent and colorimetric chemosensor for Cu2+ has been designed and fabricated. Host–guest interactions were used to construct inclusion complex magnetic nanoparticles (IFIC MNPs) from azobenzene-modified rhodamine 6G and α-cyclodextrin-modified Fe3O4@SiO2. A selective ‘turn-on’ type fluorescence enhancement and an apparent colour change from colourless to yellow-green with Cu2+ ions in the fluorescence experiments, and light yellow to pink in the UV-visible spectra, can be achieved. Good selectivity and sensitivity for Cu2+can be gained with the IFIC MNPs, with a detection limit of 2.5 × 10−7 M in MeCN/H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Furthermore, the IFIC magnetic nanoparticles can be separated and recycled easily using a magnet due to their superparamagnetism.
1. Furthermore, the IFIC magnetic nanoparticles can be separated and recycled easily using a magnet due to their superparamagnetism.
Introduction
Among the heavy metal ions, copper is a significant pollutant due to its widespread applications in industry.1,2 Pathology investigations have also shown that the abnormal concentration of copper ions in the nervous system can cause diseases such as Alzheimer’s and Parkinson’s.3 Although there have been many articles reported,4–7 it is still intriguing to develop new methodologies for the detection of copper ions in aqueous solutions with both high sensitivity and selectivity.Recent reports have shown great interest in developing chemosensors based on magnetic nanoparticles. Their good dispersibility and strong magnetic responsivity indicate potential applications in the detection and separation of metal ions.8,9 These nanoparticles have high surface areas due to their core–shell structures, composed of both inorganic and organic components. Recent studies also showed the chromogenic or fluorogenic features of these magnetic silica nanoparticles and their capacities for fluorescent sensor applications.10–13
A fascinating alternative to the preparation of functionalized magnetic chemosensors is using supramolecular interactions, which can be easily tuned by geometrically complementary host and guest molecules such as α-cyclodextrin (α-CD) (host) and azobenzene (guest).14 Being different from those that are fabricated through covalent bonds, these magnetic nanoparticle-based chemosensors can be used repeatedly. No defects, such as fluorophore leaking, lack of thickness control, inner-layer analyte diffusion, poor aqueous solubility and strict reaction conditions, have been encountered during their preparation, in contrast with traditional methods.15 So far, some reports have already put this methodology into use for application in fluorescent self-assembled inner monolayers.16
With the inspiration triggered by this neoteric method in mind, in this paper we have designed and fabricated a novel magnetic nanoparticle-based chemosensor composed of rhodamine–azobenzene-loaded magnetic nanoparticles. The fluorescent receptor, rhodamine–azobenzene, has been fastened to the surfaces of MNPs only by host–guest interactions, and the release is automatically facilitated only by changing the solvent conditions (Scheme 1).17 Moreover, the response is faster due to the direct exposure of fluorophores fabricated in such a self-assembling manner compared with those formed by covalent bonds, and the recycling of the sensing molecules and nanoparticles is facile and effective due to the magnetic nature and host–guest structure of these MNPs. It is promising that the ITCRh6G-Azo/Fe3O4@SiO2-α-CD inclusion complex magnetic nanoparticles (IFIC-MNPs) could act as a highly selective and sensitive fluorescent and colorimetric sensor for detecting trace Cu2+ ions, which can be used repeatedly.
Experimental
Materials and reagents
Rhodamine 6G (Rh6G), α-cyclodextrin (α-CD), triethylamine and 4-aminoazobenzene were purchased from Sigma-Aldrich. Thionyl chloride, ammonia, tetraethylorthosilicate (TEOS) and hydrazine hydrate were obtained from Alfa Aesar. γ-(2,3-Epoxypropoxy)propyltrimethoxysilane (KH-560, 99%) was obtained from Acros Organics. All reagents and inorganic metal salts that were of analytical grade (Shanghai Chemical Reagents Co., China) were used without further purification. The solutions of metal ions were prepared from CaCl2, CoCl2·6H2O, MgSO4, BaCl2·2H2O, CdCl2, Mn(NO3)2·6H2O, Zn(NO3)2·6H2O, NiCl2·6H2O, HgCl2, KCl, FeCl3·6H2O, CuCl2·2H2O and Pb(NO3)2 respectively, and were dissolved in deionized water. Aqueous Tris–HCl (0.05 mol L−1) solution was used as a buffer to keep pH value at 7.20, and to maintain the ionic strength of all solutions in the experiments.Apparatus
1H and 13C NMR spectra were measured on a Varian Mercury-300BB NMR spectrometer. The pH values of the test solutions were measured with a glass electrode connected to a Mettler-Toledo Instruments DELTA 320 pH meter (Shanghai, China) and adjusted if necessary. The morphology of the nanoparticles was observed on a Hitachi SU8020 scanning electron microscope. The magnetic hysteresis loops were measured on a Quantum Design SQUID-MPMS-XL magnetic property measurement system. FTIR spectra of the products were recorded on a Perkin-Elmer Paragon1000 FTIR spectrometer. HRMS was carried out with an Agilent1290–microTOF Q II (Bruker) spectrometer. Concentrations of metal ions were measured on an Agilent 7500ce inductively coupled plasma mass spectrometer. Absorption and luminescence spectra were studied on a Shimadzu Electronic UV 2100 PC UV-visible spectrophotometer and a Hitachi F-4500 luminescence spectrometer, respectively.Preparation of IFIC MNPs
The ITCRh6G-Azo/Fe3O4@SiO2-α-CD inclusion complex magnetic nanoparticles (IFIC MNPs) were easily prepared from cyclodextrin-functionalized magnetic silica microspheres (Fe3O4@SiO2-α-CD MNPs) (host) and ITCRh6G-Azo (guest) using a ‘self-assembly’ technique. The Fe3O4@SiO2-α-CD MNPs were prepared via a multistep process (Scheme 2). In brief, monodisperse superparamagnetic Fe3O4@silica spheres (denoted as Fe3O4@SiO2 MNPs) were prepared using a reported method.18 Then, a cyclodextrin-based silane coupling agent was readily synthesized according to a literature method.19 α-Cyclodextrin was linked onto the surface of the Fe3O4@SiO2 nanoparticles covalently through the reaction of the Fe3O4@SiO2 MNPs and the cyclodextrin-based silane coupling agent (CD-Si) via a grafting reaction.20,21 The preparation of the ITCRh6G-Azo moiety is described in Scheme 3. All the detailed procedures can be found in the ESI.† The synthetic product was well characterized using FTIR, SEM, XRD, a superconducting quantum interference measurement device (SQUID), UV-vis spectroscopy, 1H NMR and 13C NMR.Results and discussions
Morphology of the nanoparticles
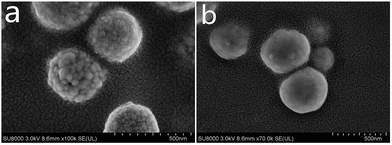
SEM images show the morphology of the Fe3O4 and Fe3O4@SiO2 nanoparticles (Fig. 1). Interestingly, it is found that the Fe3O4 nanoparticles prepared via a hydrothermal reaction possess coarse surfaces (Fig. 1a). However, after the coating process, the resulting nanostructures possess a smooth surface and are quite uniform in size (Fig. 1b). The diameter of the Fe3O4 is 350 nm and the thickness of the SiO2 shell is 50 nm. In general, we can get a thin SiO2 layer after the coating process.X-ray powder diffraction pattern analysis
In order to study the crystal structure of the nanoparticles, X-ray diffraction (XRD) experiments have been carried out (Fig. 2). In the figure, several strong reflection peaks can be seen in the 2θ region of 10–80°. We can index six diffraction peaks from Fig. 2, which are (220), (311), (400), (422), (511) and (440); these data are in agreement with the data of magnetite in the JCPDS (JCPDS Card: 19-629) file. This suggested the retention of crystalline structure after template extraction. The amorphous SiO2 shells can be proved to exist by the broad featureless XRD peak at a low diffraction angle in the XRD pattern of the iron-oxide–SiO2 core–shell nanoparticles. According to the results we can conclude that the Fe3O4 MNPs are successfully coated and passivated by the SiO2 shell.Fourier transform IR spectroscopy analysis
FTIR was carried out to investigate the bonding information of the Fe3O4@SiO2-α-CD MNPs. Fig. 3 shows the FTIR spectra of the Fe3O4@SiO2 MNPs (a), α-CD (b), Fe3O4@SiO2-α-CD MNPs (c), ITCRh6G-Azo (d) and IFIC MNPs (e). Fig. 3a and c exhibit bands at 470 cm−1, 576 cm−1 and 1090 cm−1, which are attributed to the bending vibration of Si–O–Si, symmetric Si–O–Si stretching and asymmetric Si–O–Si stretching of silane, respectively. The spectrum of the Fe3O4@SiO2-α-CD MNPs (c) differs considerably from that of the Fe3O4@SiO2 MNPs (a) in the range of 1600–3000 cm−1. Fig. 3c exhibits bands at 2925 cm−1 and 1655 cm−1, which are attributed to the alkane C–H stretching vibration and O–H bending vibration of cyclodextrin. In particular, the band at 1730 cm−1 in Fig. 3b, attributed to O–H bending, shifted to 1655 cm−1 in Fig. 3c, induced by the weakening of hydrogen bonding due to the bonding between silane and cyclodextrin, which proved the linkage of α-CD and the Fe3O4@SiO2 MNPs. In addition, bands at 1520 cm−1 (C–C stretching of benzene skeleton) and 1710 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration) can also be found in both Fig. 3d and e. Based on these data from FT-IR, it can be concluded that the resulting product, ITCRh6G-Azo/Fe3O4@SiO2-α-CD inclusion complex magnetic nanoparticles (IFIC MNPs), was doubtlessly prepared.
O stretching vibration) can also be found in both Fig. 3d and e. Based on these data from FT-IR, it can be concluded that the resulting product, ITCRh6G-Azo/Fe3O4@SiO2-α-CD inclusion complex magnetic nanoparticles (IFIC MNPs), was doubtlessly prepared.
 | ||
| Fig. 3 FT-IR spectra of Fe3O4@SiO2 MNPs (a), α-CD (b), Fe3O4@SiO2-α-CD MNPs (c), ITCRh6G-Azo (d) and IFIC MNPs (e). | ||
Magnetic property
The magnetic hysteresis loops of the Fe3O4 and IFIC MNPs measured at T = 300 K are shown in Fig. 4. The low coercivity and lack of obvious hysteresis indicate the superparamagnetism of the MNPs. The saturation magnetization (Ms) values for the Fe3O4 nanoparticles and IFIC MNPs were 83.31 and 30.06 emu g−1, respectively. The nonmagnetic materials, such as the organic ligands and silica shell, are the reason for the decrease in Ms. Also, a quenching effect is brought about by the binding of silica and ITCRh6G-Azo on the particle surface.22 In addition, the lack of complete coordination of magnetic molecules on the surface increases the surface spin disorientation.23 A decrease in the effective magnetic moment might have been caused by the disordered structure in the amorphous materials.24 However, a strong magnetic property has been transmitted from the Fe3O4 nanoparticles to the IFIC MNPs. When putting a magnet near the bottle containing the IFIC MNPs dispersed in CH3CN–H2O, the MNPs are gathered near the magnet within 9 seconds (Fig. 5). From these experiments we can conclude that the MNPs have promise to offer a simple and efficient way to extract copper ions from wastewater.The effect of pH
Protons in the testing solvents can induce great disturbance during the detection of metal ions, so it is essential to investigate the influence of pH value on the detection of Cu2+ ions by the chemosensor. A series of experiments was carried out in a pH range from 1 to 14 to avoid the precipitation problem caused by Cu(OH)2 under alkaline conditions in Cu2+ solutions. The fluorescence spectra of the IFIC MNPs were obtained with and without Cu2+ ions ([IFIC MNPs] = 0.5 g L−1, CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, excited at 345 nm, observed at 555 nm), which are shown in Fig. 6. When pH < 4, the fluorescence of the IFIC MNPs is obviously enhanced due to the protonation of the spiral lactam of rhodamine, with or without the Cu2+ ions. When pH > 12, the fluorescence recedes in the presence of Cu2+ ions (black line) and remains the same without Cu2+ due to the formation of Cu(OH)2 under alkaline conditions. In the pH value range from 7 to 12, the intensity of the fluorescence remains unchanged. Therefore, the testing solvent is buffered at 7.20.
1, v/v, excited at 345 nm, observed at 555 nm), which are shown in Fig. 6. When pH < 4, the fluorescence of the IFIC MNPs is obviously enhanced due to the protonation of the spiral lactam of rhodamine, with or without the Cu2+ ions. When pH > 12, the fluorescence recedes in the presence of Cu2+ ions (black line) and remains the same without Cu2+ due to the formation of Cu(OH)2 under alkaline conditions. In the pH value range from 7 to 12, the intensity of the fluorescence remains unchanged. Therefore, the testing solvent is buffered at 7.20.
 | ||
| Fig. 6 Fluorescence intensity of IFIC MNPs in CH3CN–H2O (1/1, v/v) with and without Cu2+ ions, measured as a function of pH. | ||
Fluorescence and UV-vis spectrum properties
Fluorescence spectra and UV-vis absorption spectra were obtained to gain insight into the signaling properties of the IFIC MNPs toward Cu2+. The fluorescence spectra with the Cu2+ ions were obtained using a solution of IFIC MNPs (0.5 g L−1) in buffered (0.05 M Tris–HCl, pH = 7.20) CH3CN–H2O (1/1, v/v). The fluorescence spectra with different concentrations of Cu2+ ions in the IFIC MNPs solution are shown in Fig. 7. Upon addition of increasing concentrations of Cu2+ ions (0 to 1.0 × 10−5 mol L−1), a significant enhancement in the characteristic fluorescence of the rhodamine 6G fluorophore moiety in a Cu2+ ion concentration-dependent manner emerges at 555 nm (excited at 345 nm), accompanied by an obvious yellow-green fluorescence enhancement. A linear relationship existed between the fluorescence intensity of the IFIC MNPs and the concentration of Cu2+ over the range of 5.0 × 10−6 to 1.0 × 10−5 mol L−1. The correlation coefficient was R = 0.99835, SD = 0.97743, k = 11.72817. The detection limit, based on the definition by IUPAC,25 was 2.5 × 10−7 mol L−1 (CDL = 3SDk−1). This value is lower than the acceptable value mandated by the EPA for the concentration of copper in drinking water (0.5 mg L−1).26 Thus, the sensor can be useful in detecting inorganic copper in samples of biological products, drugs, fish and other aqueous solutions.The UV-vis absorption spectra of the IFIC MNPs with varying Cu2+ concentrations were recorded, as shown in Fig. 8. Upon addition of Cu2+ to the IFIC MNPs (0.5 g L−1), the peak around 528 nm is significantly enhanced, suggesting the formation of the ring-opened tautomer of the rhodamine 6G moiety upon Cu2+ binding. According to the molecular structure and spectral results of the ITCRh6G-Azo moiety, it is concluded that the Cu2+ ions could chelate with the carbonyl O and thiourea S atoms and thus a ring opening of the spirolactam of rhodamine 6G took place. The hydrazine-modified rhodamine is just suitable for the Cu2+ ions in terms of steric effects, so this reaction has a high selectivity for Cu2+ ions. In this case, the solution exhibited an obvious and characteristic color change from light yellow to pink. IFIC MNPs thus can be used as a “naked eye” detector of Cu2+. The detection threshold for Cu2+ ranged from 0 to 5.0 × 10−5 mol L−1, and at this level the color change was very obvious.
Metal ion competition studies
The fluorescence emission responses of the IFIC MNPs upon addition of various biologically and environmentally relevant metal ions, namely Zn2+, Co2+, Ba2+, Mn2+, K+, Ca2+, Ni2+, Mg2+, Hg2+, Pb2+, Fe3+ and Cd2+ ions, each at a concentration of 10 μM (white bars in Fig. 9), were measured to estimate the selectivity of the IFIC MNPs as a fluorescent probe for Cu2+ ions (monitored at 555 nm, excited at 345 nm). As we expected, besides the weak effect caused by Hg2+, the above-mentioned metal ions show little effect on the fluorescence intensity of the nanosensor. However, compared with the marked enhancement produced by Cu2+ ions (5 μM), the influence of the above-mentioned metal ions is negligible.To test the practical applications of IFIC as a Cu2+-selective fluorescent sensor (monitored at 555 nm, excited at 345 nm), competition experiments were carried out to investigate the effect of other cations coexisting with Cu2+ in the presence of IFIC. The emission spectra of IFIC (0.5 g L−1) and Zn2+, Co2+, Ba2+, Mn2+, K+, Ca2+, Ni2+, Mg2+, Hg2+, Pb2+, Fe3+ or Cd2+ (10 μm) followed by addition of Cu2+ (5 μm) were recorded (black bars in Fig. 9). No obvious interference was shown by those ions in the detection for Cu2+. Thus, the IFIC MNPs exhibited excellent selectivity toward Cu2+, which makes their practical application feasible.
Adsorption experiments of metal ions onto IFIC MNPs
Adsorption kinetics experiments were carried out to investigate the adsorption ability of IFIC MNPs for Cu2+ and other metal ions in solution (Fig. 10). The IFIC MNPs were added to aqueous solutions containing Cu2+ ions from 0 to 50 mg L−1 ([IFIC MNPs] = 5 g L−1, CH3CN–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.20, 0.05 M Tris–HCl). A magnet was used to remove the magnetic nanoparticles from the solvent after 24 hours and ICP-MS to detect the concentration of Cu2+ in the solvent. Fig. 10a shows the equilibrium adsorption amounts within 24 hours under various equilibrium concentrations. It was found from Fig. 10a that the adsorption of Cu2+ increased with [Cu2+] and after 15 mg L−1 the value remained unchanged. The initial increase in adsorption may due to the vast number of coordination sites on the surface of the IFIC MNPs, and when all the sites were occupied the value leveled off. The Langmuir adsorption equation was applied to analyze the data, which is given as Ce/qe = 1/KLqm + Ce/qm, where qe is the equilibrium quantity of the metal ions adsorbed onto the IFIC MNPs (mg g−1), Ce is the equilibrium concentration (mg L−1), and qm (mg g−1) and KL (L mg−1) are the Langmuir constants related to the saturation adsorption capacity and binding energy (affinity), respectively. The Langmuir Ce/qe versus Ce plot is shown in Fig. 10b and the equation was calculated as Ce/qe = 0.16633 + 0.05489Ce. After calculation we can determine that qm = 18.22 mg g−1, KL = 0.33 L mg−1 and R2 = 0.9597, which are also shown in Table 1.
1, v/v, pH = 7.20, 0.05 M Tris–HCl). A magnet was used to remove the magnetic nanoparticles from the solvent after 24 hours and ICP-MS to detect the concentration of Cu2+ in the solvent. Fig. 10a shows the equilibrium adsorption amounts within 24 hours under various equilibrium concentrations. It was found from Fig. 10a that the adsorption of Cu2+ increased with [Cu2+] and after 15 mg L−1 the value remained unchanged. The initial increase in adsorption may due to the vast number of coordination sites on the surface of the IFIC MNPs, and when all the sites were occupied the value leveled off. The Langmuir adsorption equation was applied to analyze the data, which is given as Ce/qe = 1/KLqm + Ce/qm, where qe is the equilibrium quantity of the metal ions adsorbed onto the IFIC MNPs (mg g−1), Ce is the equilibrium concentration (mg L−1), and qm (mg g−1) and KL (L mg−1) are the Langmuir constants related to the saturation adsorption capacity and binding energy (affinity), respectively. The Langmuir Ce/qe versus Ce plot is shown in Fig. 10b and the equation was calculated as Ce/qe = 0.16633 + 0.05489Ce. After calculation we can determine that qm = 18.22 mg g−1, KL = 0.33 L mg−1 and R2 = 0.9597, which are also shown in Table 1.
| Metal ion | KL/L mg−1 | qm/mg g−1 | R2 |
|---|---|---|---|
| Cu2+ | 0.33 | 18.22 | 0.9597 |
Adsorption experiments were also carried out for other metal ions, namely Zn2+, Co2+, Ba2+, Mn2+, K+, Ca2+, Ni2+, Mg2+, Hg2+, Pb2+, Fe3+ and Cd2+, each at a concentration of 100 mg L−1 ([IFIC MNPs] = 2 g L−1, [Cu2+] = 1.0 × 10−5 mol L−1, CH3CN–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH = 7.20, 0.05 M Tris–HCl). Fig. 11 shows the adsorption amounts of each metal ion. The results showed that the IFIC MNPs can adsorb metal ions to an extent and can even reach 28 mg g−1 for Fe3+. From the results we can conclude that the IFIC MNPs show great adsorptivity for different metal ions, which may be due to the intensive distribution of α-cyclodextrin on the surface of the IFIC MNPs. The adsorption ability endows the MNPs with a promising prospect for the recycling of metal ions in solutions.
1, v/v, pH = 7.20, 0.05 M Tris–HCl). Fig. 11 shows the adsorption amounts of each metal ion. The results showed that the IFIC MNPs can adsorb metal ions to an extent and can even reach 28 mg g−1 for Fe3+. From the results we can conclude that the IFIC MNPs show great adsorptivity for different metal ions, which may be due to the intensive distribution of α-cyclodextrin on the surface of the IFIC MNPs. The adsorption ability endows the MNPs with a promising prospect for the recycling of metal ions in solutions.
 | ||
| Fig. 11 Adsorption capacity of IFIC MNPs for various metal ions (CH3CN–H2O, 1 : 1, v/v, buffered at pH 7.20 with 0.05 M Tris–HCl, [Cu2+] = 1.0 × 10−5 mol L−1, metal ions: 100 mg L−1, MNPs: 2 g L−1). | ||
Regeneration efficiency of the sensing system
Recycling of the IFIC nanoparticles was conducted for detection of copper ions (Fig. 12). After each cycle, EDTA was added into the testing solvent to bind Cu2+ in order to make the complex dissociate. After magnetic separation, the IFIC nanoparticles were washed ultrasonically with DMF at 50 °C; this procedure can destroy the self-assembled structure, which is magnetically separated again, dried under vacuum, and reused in the system of a solution of ITCRh6G-Azo moieties to rebuild the host–guest moieties. The recycling was easy and high yielding. Fig. 12 illustrates the fluorescence intensity of the IFIC–Cu2+ in each cycle ([Cu2+] = 1.0 × 10−5 mol L−1). After 5 cycles, the ability to detect the Cu2+ ions is still active and efficient. Through this procedure, we can recycle the dye molecule and the magnetic nanoparticles; these can be reused in the next experiment or test. This recyclable feature can’t be achieved in covalent bonding testing systems for metal ions. | ||
| Fig. 12 Recycling and reuse of the IFIC MNP sensors for Cu2+ (CH3CN–H2O, 1 : 1, v/v, buffered at pH 7.20 with 0.05 M Tris–HCl, [Cu2+] = 1.0 × 10−5 mol L−1, at 555 nm). | ||
Conclusions
In conclusion, a new type of fluorescent chemosensor for efficiently sensing and separating Cu2+ in aqueous solution has been prepared. It has a core–shell Fe3O4@SiO2-α-CD structure loaded with ITCRh6G-Azo moieties via host–guest interactions. These multifunctional nanoparticles exhibit a high selectivity and an excellent high sensitivity for targeting copper ions over a number of other metal ions tested, with the detection limit found to be 2.5 × 10−7 mol L−1. Furthermore, the recycling of the IFIC nanoparticles is easy and high-yielding and the described approach (based on grafting reaction and self-assembly techniques) is simple and effective. It proved to be a promising alternative for developing high-performance fluorescent sensing materials for copper ion detection in aqueous solution with both high sensitivity and selectivity.Acknowledgements
We thank the National Natural Science Foundation of China (no. 21174052), the Natural Science Foundation of Jilin Province of China (no. 20130101024JC) and Jilin Provincial Science & Technology Department (no. 20140204054GX) for their generous financial support.Notes and references
- S. P. Wu, R. Y. Huang and K. J. Du, Dalton Trans., 2009, 4735–4740 RSC.
- R. Krämer, Angew. Chem., Int. Ed., 1998, 37, 772–773 CrossRef.
- C. Deraeve, C. Boldron, A. Maraval, H. Mazarguil, H. Gornitzka, L. Vendier, M. Pitie and B. Meunier, Chem. Eur. J., 2008, 14, 682–696 CrossRef CAS PubMed.
- B. L. Ma, S. Z. Wu and F. Zeng, Sens. Actuators. B., 2010, 145, 451–456 CrossRef CAS PubMed.
- Z. J. Chen, L. M. Wang, G. Zou, J. Tang, X. F. Cai, M. S. Teng and L. Chen, Spectrochim. Acta. A., 2013, 105, 57–61 CrossRef CAS PubMed.
- Y. Xiang, A. J. Tong, P. Y. Jin and Y. Ju, Org. Lett., 2006, 8, 2863–2866 CrossRef CAS PubMed.
- M. Dong, T. H. Ma, A. J. Zhang, Y. M. Dong, Y. W. Wang and Y. Peng, Dyes. Pigments., 2010, 87, 164–172 CrossRef CAS PubMed.
- Y. Ma, B. Z. Zheng, Y. Zhao, H. Y. Yuan, Y. Q. Cai, J. Du and D. Xiao, Biosens. Bioelectron., 2013, 48, 138–144 CrossRef CAS PubMed.
- Q. He, E. W. Miller, A. P. Wong and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9316–9317 CrossRef CAS PubMed.
- J. H. Jung, J. H. Lee and S. Shinkai, Chem. Soc. Rev., 2011, 40, 4464–4474 RSC.
- C. Wang, S. Tao, W. Wei, C. Meng, F. Liua and M. Hana, J. Mater. Chem., 2010, 20, 4635–4641 RSC.
- N. Insin, J. B. Tracy, H. Lee, J. P. Zimmer, R. M. Westervelt, M. G. Bawendi, R. M. Westervelt and M. G. Bawendi, ACS Nano, 2008, 2, 197–202 CrossRef CAS PubMed.
- R. Hao, R. J. Xing, Z. C. Xu, Y. L. Hou, S. Gao and S. H. Sun, Adv. Mater., 2010, 22, 2729–2742 CrossRef CAS PubMed.
- J. S. Yang and T. Swager, J. Am. Chem. Soc., 1998, 120, 5321–5322 CrossRef CAS.
- D. R. Bae, W. S. Han, J. M. Lim, S. W. Kang, J. Y. Lee, D. M. Kang and J. H. Jung, Langmuir, 2010, 26, 2181–2185 CrossRef CAS PubMed.
- H. J. Kim, M. Lee, H. L. Mutihac, J. Vicens and J. S. Kim, Chem. Soc. Rev., 2012, 41, 1173–1190 RSC.
- R. A. Potyrailo, R. C. Conrad, A. D. Ellington and G. M. Hieftje, Anal. Chem., 1998, 70, 3419–3425 CrossRef CAS.
- S. Wang, Q. W. Liu, Y. Zhang, S. D. Wang, Y. X. Li, Q. B. Yang and Y. Song, Appl. Surf. Sci., 2013, 279, 150–158 CrossRef CAS PubMed.
- X. Xu, C. Deng, M. Gao, W. Yu, P. Yang and X. Zhang, Adv. Mater., 2006, 18, 3289–3293 CrossRef CAS PubMed.
- X. Y. Ling, I. Y. Phang, G. J. Vancso, J. Huskens and D. N. Reinhoudt, Langmuir, 2009, 25, 3260–3263 CrossRef CAS PubMed.
- D. Zhang and J. Chang, Adv. Mater., 2007, 19, 3664–3667 CrossRef CAS PubMed.
- S. Ghosh, A. Z. M. Badruddoza, M. S. Uddin and K. Hidajat, J. Colloid Interface Sci., 2011, 354, 483–492 CrossRef CAS PubMed.
- D. H. Chen and S. H. Wu, Chem. Mater., 2000, 12, 1354–1360 CrossRef CAS.
- R. H. Kodama, A. E. Berkowitz, E. J. McNiff and S. Foner, J. Appl. Phys., 1997, 81, 5552–5557 CrossRef CAS PubMed.
- H. M. N. H. Irving, H. Freiser and T. S. West, IUPAC Compendium of Analytical Nomenclature, Definitive Rules, Pergamon Press, Oxford, 1981 Search PubMed.
- Guidelines for Drinking-Water Quality, World Health Organization, Geneva, 1996 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: experimental details, additional spectroscopic data. See DOI: 10.1039/c5ra11251f |
| This journal is © The Royal Society of Chemistry 2015 |