Enhanced reductive transformation of 2,4-dinitroanisole in a anaerobic system: the key role of zero valent iron†
Changjin Oua,
Shuai Zhangab,
Jianguo Liua,
Jinyou Shen*a,
Weiqing Hana,
Xiuyun Suna,
Jiansheng Lia and
Lianjun Wang*a
aJiangsu Key Laboratory for Chemical Pollution Control and Resources Reuse, School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing, 210094, China. E-mail: shenjinyou@mail.njust.edu.cn; wanglj@mail.njust.edu.cn; Fax: +86 25 84303965, +86 25 84315941; Tel: +86 25 84303965, +86 25 84315941
bJiangsu Changhuan Environmental Science Co. LTD, Changzhou 213022, Jiangsu Province, China
First published on 1st September 2015
Abstract
Accelerated reduction of typical multi-substituted nitroaromatic compounds (NACs), i.e., 2,4-dinitroanisole (DNAN), was achieved in an anaerobic system coupled with zero valent iron (ZVI), with the underlying role of ZVI in this process elucidated. Both removal of DNAN and formation of its final reductive product 2,4-diaminoanisole (DAAN) were notably improved in the ZVI coupled biosystem. In the ZVI coupled biosystem and biotic control system, complete removal of DNAN could be achieved within 4 h and 20 h, respectively. However, only 28.71 ± 5.06% of DNAN could be removed in the ZVI control system after 20 h. Correspondingly, the formation efficiencies of DAAN in the ZVI coupled biosystem, biotic control system and ZVI control system were 99.66 ± 0.70%, 16.99 ± 1.73% and 0.00 ± 0.00%, respectively. The increased DNAN removal and DAAN formation in the ZVI coupled biosystem was linked to the high accumulation of formate, low oxidation–reduction potential (ORP) and great pH self-buffering capability, which was provided by the addition of ZVI. Compared with the biotic control system, the production of CH4 was significantly accelerated in the ZVI coupled biosystem, indicating that a favorable environment for methanogens was created at the presence of ZVI. Especially, the ZVI coupled biosystem displayed a more stable performance in terms of DNAN reduction with the coexistence of the competitive electron acceptors, such as nitrate and sulfate. Therefore, the ZVI coupled biosystem could be a promising alternative to the conventional anaerobic reduction process for the removal of multi-substituted NACs from wastewater.
1. Introduction
2,4-Dinitroanisole (DNAN) is an important ingredient in the production of dyes and insecticides.1 Recently, DNAN is being considered as a replacement for sensitive explosives such as 2,4,6-trinitrotoluene (TNT), because of its insensitive properties.2 Compared to TNT, the detonation temperature of DNAN is higher, which is beneficial for manufacture, transport and store processes.3 However, considering its potential environmental risk, high toxicity, poor biodegradability and wide usage, improper disposal of DNAN containing waste can lead to tremendous environmental pollution, including water contamination and soil problems. Due to the pronounced electron-withdrawing character of the nitro groups on the benzene ring, nitroaromatic compounds (NACs) harbors a highly electron deficient π-electron system, resulting into the difficulty in chemical oxidation or biological oxidation.4 Moreover, with the increase of the nitro group number, mineralization of multi-substituted NACs such as DNAN through oxidative pathways becomes more resistant.5 Thus, there are significant needs of appropriate methods for the remediation of the sites contaminated by DNAN.Under anaerobic or anoxic conditions, NACs succumb to electrophilic attack and can be transformed to their corresponding aromatic amines but without cleaving the aromatic ring. Generally, the produced aromatic amines are less toxicity and easier to mineralize than their parent compounds.6 Nevertheless, due to the highly recalcitrant and toxicological nature of NACs, the anaerobic reduction is usually limited by low degradation rate and poor stability. Therefore, it is important to improve anaerobic reduction performance to achieve more effective reduction of NACs such as DNAN.
Zero valent iron (ZVI) is currently attracting wide interest in the treatment of wastewater and groundwater due to its inexpensive, reliable and moderately strong reduction properties. Some refractory contaminants at oxidative state, such as NACs, azo dyes and halogenated organic compounds, could be effectively reduced in the ZVI process.7–9 For the treatment of the wastewater containing these refractory contaminants, ZVI reduction process is often used prior to the biological process for reducing toxicity and improving biodegradability.4,10 Ahn et al.11 reported that the iron pretreatment not only removed energetic compounds but also eliminated the toxic effect on perchlorate reducing bacteria. Oh et al.12 also showed that the ZVI pretreatment transformed recalcitrant hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) to ring-opening products, i.e., formaldehyde, which are more amenable to mineralization by aerobic bacteria. Therefore, the combined ZVI and biological process offers bright prospects for the treatment of highly recalcitrant industrial wastewater.13 In general, ZVI process and biological process was often operated in sequence, however, coupling of ZVI into the biological process could facilitate the degradation of pollutants in a single reactor, which might take full advantage of both ZVI and biological process.14 Considering its reductive property, ZVI is expected to be helpful for creating an enhanced anaerobic environment which may be beneficial to improve the performance of an anaerobic reactor in wastewater treatment.15 Meanwhile, ZVI corrosion products, especially alkaline byproducts Fe(OH)2 or Fe(OH)3, can not only act as the acid buffers, but also provide another alternative for the contaminant removal through flocculation, adsorption and precipitation.16
Given these, attentions have been increasingly paid to the combined use of ZVI and microbe for enhanced degradation of recalcitrant contaminants from wastewater.8,17 Liu et al.18 reported that both azo dye decolorization and COD removal were remarkably improved in an acidogenic reactor packed with ZVI. At the presence of ZVI, the abundance of methanogens was significantly increased and microbial strains responsible for azo dye decolorization were enriched in the anaerobic reactor.15 Of even greater importance, the release of H2 during ZVI corrosion became an alternative electron donor for hydrogen-consuming microorganisms, such as methanogenic and denitrifying bacteria, as well as some reduction related species.19 Even though these physicochemical and microbial interactions are highly important for the overall performance of the coupled system, systematic investigation on the ZVI coupled anaerobic reduction system is still limited. In addition, coupling of ZVI into an anaerobic biological system for the treatment of multi-substituted NACs containing wastewater has been rarely investigated, and the underlying role of ZVI in the coupled system treating NACs containing wastewater is not fully understood.
Therefore, in this study, coupling of ZVI into the anaerobic system was established with the goal of accelerating the DNAN removal from wastewater. Specially, the key role of ZVI in the coupled system was investigated in terms of the intermediate products, ORP, pH and biogas analysis. The performance of the ZVI coupled biosystem at the presence of competitive electron acceptors, such as nitrate and sulfate, was also evaluated.
2. Materials and methods
2.1 Chemicals
DNAN was a gift from Hubei Dongfang Chemical Co. Ltd in Hubei province, China. 2-Nitro-4-aminoanisole (2-N-4-AAN) and 2-amino-4-nitroanisole (2-A-4-NAN) were purchased from Bepharm Co. Ltd (Shanghai, China). DAAN was purchased from Sun Chemical Technology Co. Ltd (Shanghai, China). ZVI powder with analytical purity was purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China) and was used without pretreatment.2.2 Synthetic wastewater and sludge cultivation
The composition of the synthetic wastewater used in this study was as follows: DNAN (50 mg L−1), methanol (0.84 mL L−1), KH2PO4 (25 mg L−1), NH4Cl (100 mg L−1), MgSO4·7H2O (200 mg L−1), CaCl2 (30 mg L−1), and trace element solution (SL-4, 10 mL L−1). In order to give sufficient electron donor for DNAN reduction, methanol was added excessively. The composition of SL-4 was as described previously by Shen et al.20Anaerobic sludge taken from an anaerobic baffled reactor treating real NACs containing wastewater was used as the seed sludge. Before inoculation, the seed sludge was acclimated for about three months using the synthetic wastewater as the influent. Once stable reduction performance of the acclimation system was achieved, the acclimatized sludge could be used as the inoculum.
2.3 Experimental procedure
In this study, DNAN reduction was performed in batch mode, which was carried out in a series of 100 mL serum bottles. 80 mL synthetic wastewater containing 50 mg L−1 DNAN was added into each serum bottle. To remove any residual dissolved oxygen, the synthetic wastewater was purged with nitrogen for at least 20 min, followed by the addition of the 0.1 g ZVI and 20 mL seed sludge prepurged with nitrogen. The initial DNAN and MLSS concentrations in each serum bottle were calculated to be 40 mg L−1 and 13 g L−1, respectively. Then, the serum bottles were sealed with polytetrafluoroethylene/silica plugs and aluminum crimp seals. All serum bottles were incubated on a rotary shaker at 200 rpm and 30 °C. At every predetermined sampling time, one serum bottle was sacrificed, and 10 mL of the solution was filtered through a 0.22 μm membrane for analysis. The control systems, i.e., the ZVI control system with the addition of 0.1 g ZVI but without sludge, and the biotic control system with the addition of 20 mL anaerobic sludge but without ZVI, were operated according to the same experimental procedures as the coupled system.To evaluate the competitive effect of other electron acceptors on the microbial transformation of DNAN, two common competing electron acceptors, i.e., nitrate and sulfate, were added respectively to the batch anaerobic reactors at the concentration of 500 mg L−1. DNAN reduction performance at the presence of nitrate and sulfate was evaluated.
All experimental runs were performed in triplicate and the results were reported as an average of the three independent determinations.
2.4 Analytical methods
DNAN and its intermediate products were identified and quantified by high performance liquid chromatography (HPLC) (Waters 2996, Waters Incorporation, USA). The HPLC analysis was conducted at room temperature using a RP18 column (5 mm, 4.6 × 250 mm) and a UV-vis detector. The mobile phase was a mixture of 45% methanol and 55% water pumped at a flow rate of 1.00 mL min−1. The analysis was performed at 254 nm with a column temperature of 35 °C. The analysis of formate ion was performed on an ion chromatograph (ICS-2100, DIONEX, USA) using an Ion Pac® As11-HC (4 × 250 mm) column and a suppressed conductivity detector. The pH and oxidative-reductive potential (ORP) were measured by a pH meter (FE20K, Mettler-Toledo instruments, CH) with a redox electrode. At given time intervals, the volume of biogas produced was measured using a syringe after the gas pressure in the headspace was brought to atmospheric pressure. The composition of biogas was analyzed by gas chromatography (Agilent 6820, Agilent Technologies, USA) equipped with a thermal conductivity detector (TCD) using molecular sieve 5A-60/80 mesh column (ANPEL Laboratory Technologies Inc., Shanghai, China) as a separation column. N2 was the carrier gas, and the operating temperature of the injection port, oven, and detector was 150 °C, 60 °C and 200 °C, respectively. Iron concentration in the reactors was determined by inductively coupled plasma (Optima 7000, PerkinElmer instruments, USA). Scanning electron microscopy coupled with energy dispersive spectroscopy (SEM-EDS) (Quanta 250FEG, FEI, USA) was applied to characterize the morphology and chemical composition of anaerobic sludge after ZVI treatment.2.5 Data analysis
DNAN reduction rates in the reduction systems were described by the pseudo zero-order kinetic model (eqn (1)) and pseudo first-order kinetic model (eqn (2)), respectively.| C0 − Ct = k0t | (1) |
| ln(C0/Ct) = k1t | (2) |
3. Results and discussion
3.1 Performance of DNAN reduction in the ZVI coupled biosystem
To verify whether the anaerobic reduction of DNAN could be enhanced by ZVI, removal of DNAN and formation of its reduction intermediates in ZVI coupled biosystem, ZVI control system and biotic control system, were compared. As shown in Fig. 1a, only 4 h was required for complete DNAN reduction in the ZVI coupled biosystem, while as long as 20 h was required for complete DNAN reduction in the biotic control system. The difference in terms of DNAN removal was significant, probably due to the key role of ZVI in DNAN reduction. However, only 28.71 ± 5.06% of the total DNAN could be removed in the ZVI control system after 20 h, indicating that the ZVI alone could not serve as an efficient and sufficient electron donor for abiotic reduction of DNAN, especially at the neutral pH condition adopted in this study. Therefore, it could be inferred that there existed some synergistic effects between ZVI and anaerobic sludge for DNAN reduction. | ||
Fig. 1 Concentration evolution of DNAN and its corresponding reduction intermediates as a function of reduction time ( ZVI coupled biosystem, ZVI coupled biosystem,  biotic control system, biotic control system,  ZVI control system). ZVI control system). | ||
Under anaerobic condition, DNAN could be reductively transformed into DAAN with 2-amino-4-nitroanisole (2-A-4-NAN) and 2-nitro-4-aminoanisole (2-N-4-AAN) as the intermediates,2 which was confirmed by HPLC analysis (Fig. S1†). In the ZVI coupled biosystem and biotic control system, the maximum accumulation concentrations of 2-A-4-NAN and 2-N-4-AAN were 9.65 ± 1.09 mg L−1 and 2.40 ± 0.15 mg L−1, 23.11 ± 0.77 mg L−1 and 3.88 ± 0.22 mg L−1, respectively, indicating the relatively low accumulation of reduction intermediates in the ZVI coupled biosystem (Fig. 1b and c). However, only 3.65 ± 0.62 mg L−1 2-A-4-NAN was detected in the ZVI control system (Fig. 1b). Moreover, the maximum concentration of final product DAAN in the ZVI coupled biosystem was as high as 27.79 ± 0.69 mg L−1, which was much higher than 4.74 ± 0.48 mg L−1 in the biotic control system and 0.00 ± 0.00 mg L−1 in the ZVI control system (Fig. 1d). Correspondingly, the formation efficiencies of DAAN in ZVI coupled biosystem, biotic control system and ZVI control system were 99.66 ± 0.70%, 16.99 ± 1.73% and 0.00 ± 0.00%, respectively. It could be seen that DNAN was only partially reduced in the biotic control system, with more 2-A-4-NAN accumulated but less DAAN produced. These results further indicated that coupling of ZVI into the anaerobic system could accelerate the degradation of DNAN, particularly the formation of its final reductive product DAAN. In addition, accounting for these intermediate species and end products gave good mass balance (greater than 85%) for the three individual batch systems, suggesting that other reaction products were negligible.
3.2 Reduction pathway of DNAN in the ZVI coupled biosystem
Based on the evolution of intermediate products, it is interesting to note that the reduction of –NO2 on DNAN was preferential at ortho position in the ZVI coupled biosystem, resulting in the formation of 2-A-4-NAN, which could be subsequently reduced to DAAN. This phenomenon was in accordance with the result in the previous study. Olivares et al.21 also found that the reduction of the nitro groups on DNAN under anaerobic condition followed the order of ortho-position > para-position. According to the density function theory computations analysis, the charge densities of the N atoms at para-NO2 and ortho-NO2 positions of DNAN were 0.211 and 0.215, following the order of ortho-position > para-position. Electron attacks, which were nucleophilic, would occur preferentially at the N atom with more positive charge density. Therefore, the reduction of DNAN was selectively favored at the ortho-NO2 group.3.3 Kinetics of DNAN reduction in the coupled ZVI anaerobic system
Pseudo first-order and pseudo zero-order kinetic models were employed to elucidate the DNAN reductive transformation process in the three individual systems (i.e., ZVI coupled biosystem, biotic control system and ZVI control system). The rate constant and regression coefficient (R2) relevant to the zero and first kinetic models were shown in Table 1. It was noteworthy that the removal of the DNAN in either ZVI coupled biosystem or biotic control system could be appropriately simulated by the pseudo first-order kinetic model, while the removal of DNAN in ZVI control system followed the pseudo zero-order kinetic model. This result suggested that there existed different mechanisms between biotic system and abiotic system for DNAN removal.| Experiment condition | Pseudo zero-order kinetics | Pseudo first-order kinetics | ||
|---|---|---|---|---|
| k0 (mg h−1) | R2 | k1 (h−1) | R2 | |
| ZVI coupled biosystem | 9.382 | 0.943 | 1.263 | 0.951 |
| Biotic control system | 1.910 | 0.867 | 0.217 | 0.993 |
| ZVI control system | 0.625 | 0.990 | 0.018 | 0.988 |
For the solid–liquid heterogeneous reaction system, such as the ZVI reduction system or the ZVI coupled biosystem, if the adsorption of contaminants onto the solid surface played a minor role in the reductive process, the contaminant removal often followed the pseudo zero-order kinetic model, otherwise pseudo first-order kinetic model was more appropriate for the removal kinetics.22,23 Since no removal of DNAN was observed at the initial stage in the ZVI control system (Fig. 1a), the removal of DNAN in ZVI control system could be attributed to the reduction by ZVI rather than adsorption by ZVI. As a result, the pseudo zero-order kinetics model could be applied to the DNAN removal process in the ZVI control system.23,24 However, a sharp decrease of DNAN concentration was observed in either ZVI coupled biosystem or biotic control system within the first hour (Fig. 1a), probably due to the strong adsorption of DNAN by the sludge inoculated in these two systems, which was confirmed by the good match between DNAN removal and first-order kinetic in either ZVI coupled biosystem or biotic control system.25
As was indicated in Table 1, the pseudo first-order rate constant for DNAN removal in the ZVI coupled biosystem was as high as 1.263 h−1, which was much higher than 0.217 h−1 in the biotic control system. This result strongly confirmed that the removal of DNAN in anaerobic system could be largely promoted by the addition of ZVI, probably due to the synergistic interaction between anaerobic microbes and ZVI. However, the surface adsorption by ZVI was negligible, since the ZVI used in this study had few surface sites amenable for DNAN adsorption, as was indicated by the slight removal of DNAN at the early stage in the ZVI control system.
3.4 The key role of ZVI in the coupled system
As indicated in previous study, pH is one of the most important parameters affecting the community structure and activity of anaerobic microorganisms.14 After the 20 h reaction, the pH in the biotic control system and ZVI coupled biosystem shifted from 7.12 ± 0.01 to 6.35 ± 0.04 and 6.67 ± 0.02 (Table 2), respectively, suggesting that the ZVI coupled biosystem seemed to have a greater pH self-buffering capability than the biotic control system. This phenomenon could be linked to the ZVI corrosion in the anaerobic system, which consumed the acidity produced from the anaerobic acidification process.| Parameter | Influent of wastewater | Effluent of biotic control system | Effluent of ZVI control system | Effluent of ZVI coupled biosystem |
|---|---|---|---|---|
| a n.d. means not detectable. | ||||
| pH | 7.12 ± 0.01 | 6.35 ± 0.04 | 7.41 ± 0.01 | 6.67 ± 0.02 |
| Fe2+(mg L−1) | n.d. | n.d. | 0.06 ± 0.01 | 0.34 ± 0.02 |
Furthermore, ZVI corrosion process could create a more stable and favorable anaerobic environment for microorganisms by lowering the ORP.15 As shown in Fig. 2, the ORP in the ZVI coupled biosystem approximately ranged from −128.5 ± 12.0 mV to −265.0 ± 19.8 mV, while it ranged from −122.5 ± 3.5 mV to −215.5 ± 20.5 mV in the biotic control system. Lower ORP value means a better reductive environment, which could exert a positive effect on the reduction of nitro group.26,27 Additionally, a sharp decrease of ORP was observed in ZVI coupled biosystems within the first 4 h, implying that there was a substantial depletion of the oxidative compounds in aqueous solution. Such a phenomenon was well in agreement with the DNAN reduction in the ZVI coupled biosystem, confirming the effective reduction of DNAN in the ZVI coupled system.
To further clarify the effect of ZVI on methanol metabolism, the production of the methanol metabolism product, i.e., formate, was investigated. As shown in Fig. 3, the production of formate in ZVI coupled biosystem was significantly higher than that in the biotic control system. A previous work showed that both acidogenesis and activity of fermentative bacteria could be effectively improved by lowering ORP, which was provided by the addition of ZVI.28 On the other hand, the ferrous ions from ZVI corrosion could stimulate the synthesis of key enzymes in the hydrolysis-acidification process, resulting in the accumulation of volatile fatty acids.29 Considering that formate was an effective electron donor for the reduction process,30 the increased production of formate in the ZVI coupled biosystem could be beneficial for the efficient reduction of DNAN.
Generally, methanol as well as the ZVI in anaerobic system may serve as precursors for the formation of an intermediate H2 pool, which could be utilized as the electron donor for the reduction process.19,31 However, no hydrogen was produced in either biotic control system or ZVI coupled biosystem (Fig. 4). This might be attributed to the slow corrosion rate of ZVI and high consumption rate of methanol in the anaerobic system. Under anaerobic conditions, the accumulated VFAs could be further bioconverted to methane. It was observed that the cumulative CH4 in the ZVI coupled biosystem was about 0.638 ± 0.017 mmol per mmol methanol, while, it was only 0.017 ± 0.002 mmol per mmol methanol in the biotic control system, indicating that the methanogenic activity of the sludge could be effectively improved by addition of ZVI. Previous studies have shown that an appropriate amount of ferrous ions released from ZVI corrosion could be involved in energy metabolism as a cytochrome and ferredoxin in methylotrophic methanogens.32,33 Meanwhile, the CO2 produced in ZVI coupled biosystem could be further converted to methane through methanogenesis using ZVI as the direct electron donor, which was necessarily beneficial for the increase of methane production.34 Furthermore, the rapid reduction of DNAN in the ZVI coupled biosystem alleviated the inhibitive effect of DNAN on methanogens, since DNAN was much more toxic to methanogenic microorganisms than its reduction products.6,35
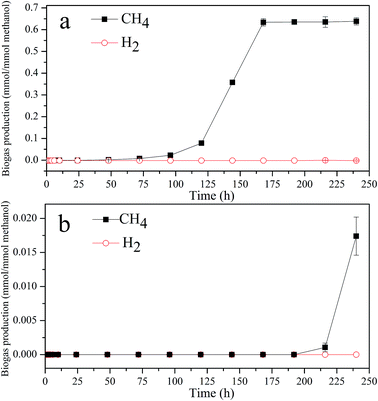 | ||
| Fig. 4 Dynamics of biogas production in the ZVI coupled biosystem (a) and biotic control system (b). | ||
Additionally, the electronegative anaerobic bacteria could be easily attached on the surfaces of ZVI due to the static function in a mixed anaerobic culture, and a stable ZVI–microbial zoogloea could be gradually formed, which was beneficial for NACs reduction.36 The SEM-EDS analysis confirmed the presence of Fe element on the outer layer and inner parts of anaerobic granules, indicating that ZVI could be a ideal site for the formation of ZVI–microbial zoogloea complex (Fig. S2†). Moreover, under anaerobic condition, the ZVI surface area might be increased by etching and pitting through corrosion, which was further beneficial for mass transfer and reductive transformation of pollutants on it.36
3.5 DNAN reduction at the presence of competitive electron acceptors
Previous studies suggested that the competitive electron acceptors in wastewater, such as nitrate and sulfate, have profound impact on the anaerobic reduction of pollutants, such as nitrobenzene, pentachloroaniline and 4-chloronitrobenzene, etc.26,31,37 Therefore, it was essential to investigate the DNAN reduction at the presence of these competitive electron acceptors. | ||
Fig. 5 Effects of nitrate on DNAN reduction as a function of time ( ZVI coupled biosystem, ZVI coupled biosystem,  ZVI coupled biosystem with nitrate, ZVI coupled biosystem with nitrate,  biotic control system, biotic control system,  biotic control system with nitrate). biotic control system with nitrate). | ||
In the biotic control system, the inhibitory effect of nitrate on DNAN removal could be expected considering the much higher oxidation potential of nitrate compared to DNAN. The standard electrode potentials for the reduction of NO3− to NO2− at neutral pH were reported to be 0.43 V vs. standard hydrogen electrode (SHE), while the one-electron reduction of DNAN was as low as −0.40 V.38–40 As a result, nitrate reduction had an advantage over DNAN reduction in the competition for the limited electron donor. The ORP increase in the anaerobic system after the addition of nitrate was another reason for the decreased DNAN removal (Fig. S3a†). However, at the presence of ZVI, the situation was different. The standard electrode potential of NO3−/NO2−, i.e., 0.43 V, was higher than that of Fe2+/Fe, i.e., −0.44 V. Therefore, the competitive electron acceptor, i.e., nitrate, could be theoretically reduced by ZVI in ZVI coupled biosystem.41 As was reported in previous study, at the presence of nitrate or nitrite, corrosion of iron might be alleviated, especially under neutral or alkaline condition.42,43 However, in this ZVI coupled biosystem, slightly acidic condition was well maintained, probably due to the acidification reaction in this anaerobic system. Therefore, corrosion of ZVI would make an important contribution for both nitrate reduction and DNAN reduction. In addition, ZVI surface area could be increased by etching and pitting through anaerobic corrosion, which was further beneficial for mass transfer and reductive reduction on it.36 More importantly, at near-neutral pH condition, nitrate as a less strong oxidant could oxidize ZVI to form the magnetite,44 overcoming the obstacle from the electron transfer barrier over the corrosion coating, which might be beneficial for NACs reduction.45 Therefore, the ZVI coupled biosystem showed excellent performance in terms of DNAN reduction at the presence of nitrate.
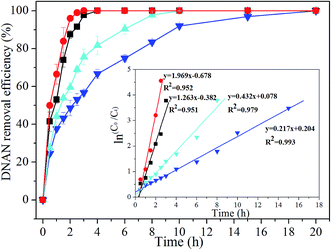 | ||
Fig. 6 Effects of sulfate on DNAN reduction as a function of time ( ZVI coupled biosystem, ZVI coupled biosystem,  ZVI coupled biosystem with sulfate, ZVI coupled biosystem with sulfate,  biotic control system, biotic control system,  biotic control system with sulfate). biotic control system with sulfate). | ||
Since the inhibitory effect of sulfate on NACs degradation had been well recognized,26 the phenomenon observed in this study was rather interesting. The reason could be ascribed to the generation of some reducing agents, e.g., sulphide, which could be used as the electron donor for DNAN. Similar result was also reported by van der Zee et al.,46 where the reduction of azo dye could be significantly enhanced at the presence of sulfate, especially under the anoxic condition. The standard electrode potential of SO42−/HSO3− was −0.52 V vs. SHE at neutral pH,38 which was lower than the one-electron standard reduction potential of DNAN. Compared with sulfate, DNAN was more subjected to reduction in this study, as DNAN showed higher competitiveness for the electron. Besides, another compelling evidence hypothesized by Ismail and Pavlostathis,31 was that the growth of sulfate reducers was relatively slow when methanol was used as the energy source. Therefore, the electron donors used for the reduction of sulfate could be limited, which was further beneficial for DNAN reduction. Meanwhile, the poisonous effect of sulphide on microorganisms might be ignored under near-neutral pH condition.47 More importantly, the iron oxides and hydroxides on the surface of ZVI could be eliminated by sulphide, with the formation of mackinawite or pyrite.48,49 Compared with the iron oxides and hydroxides, the mackinawite or pyrite was a better promoter of electron transfer to organic pollutants.50 Unfortunately, the quantification of sulphide and elemental sulfur during DNAN reduction has been unsuccessful in this study. This might be due to the low concentrations of the sulphide and elemental sulfur in the anaerobic system, which needs further investigation.
Additionally, the ORP in either biotic control system or ZVI coupled biosystem was significantly decreased at the presence of sulfate, indicating that a more reductive condition was created for DNAN reduction (Fig. S3b†). In terms of the fore-mentioned discussion, it could be concluded that the DNAN removal was accelerated by addition of sulfate in either ZVI coupled biosystem or biotic control system.
3.6 Implication of this work
Compared to the biotic control system and ZVI control system, both DNAN reduction and DAAN formation were significantly improved in the ZVI coupled biosystem. The efficient reduction of DNAN to DAAN in the ZVI coupled biosystem would result in a significant improvement of biodegradability and reduction of toxicity.51 More importantly, favorable environment for some specific microbial species, such as methanogens, could be created by offsetting the possible pH decline and lowering the ORP.What's more, the Fe2+ in the effluent of the ZVI coupled biosystem was generally below 1 mg L−1, suggesting the slow rate of ZVI dissolution. The low consumption of iron leads to easy maintenance and low operating cost. In addition, the low concentration of ferrous iron was beneficial for the growth of microorganisms.32,33 Moreover, under anaerobic condition, ZVI could be protected from oxygen, with the reduced formation of iron oxides on the surface.15 Thus the frequent replacement and regeneration of ZVI was not required in the ZVI coupled biosystem. What's more important, under anaerobic condition, ZVI surface area could be increased by etching and pitting through corrosion, which was further beneficial for mass transfer and reductive reduction on it.36
Recently, for the removal of various contaminants, the application of ZVI powder, especially the nano zero valent iron (NZVI), has received increasing attention due to their high surface area and high reactivity. However, coupling of ZVI powder into an anaerobic system for the treatment of raw industrial wastewater was limited by far, due to the inherent weakness of ZVI powder and NZVI, such as poor stability and easy aggregation. To address these issues, iron shavings may be a better choice, compared with ZVI powder and NZVI. The primary reason for this selection was the abundant local supply, relatively low cost and fairly large surface area. Ma and his co-workers have undertaken a major research and development project to investigate the technical and economic feasibility of iron shavings for the enhance treatment of industrial process wastes, with success achieved.10 Nowadays, coupling of iron shaving into the upflow anaerobic sludge blanket (UASB) has been developed in our laboratory for the treatment of high strength wastewater containing NACs. The interaction between iron shaving and microorganisms, as well as the dynamic change of iron surface and microbial population after long-term operation, will be investigated in our future study.
4. Conclusions
Compared to the biotic control system and the ZVI control system, both DNAN reduction and DAAN formation were significantly improved in the ZVI coupled biosystem. The high performance of the ZVI coupled biosystem could be attributed to the high accumulation of formate, low ORP and great pH self-buffering capability at the presence of ZVI. Compared with the biotic control system, the survival environment for methanogens was effectively improved in ZVI coupled biosystem. In addition, the ZVI coupled biosystem showed high efficiency in terms of DNAN removal with the coexistence of competitive electron acceptors, such as nitrate and sulfate. The ZVI coupled biosystem could be a promising alternative to the conventional anaerobic reduction process for the removal of recalcitrant contaminants from wastewater, especially for the treatment of wastewater containing multi-substituted NACs.Acknowledgements
This research is financed by Innovation Program of Foundation Product, Major Project of Water Pollution Control and Management Technology of P. R. China (No. 2012ZX07101-003-001), Zijin Intelligent Program of NJUST (No. 2013-ZJ-02-19) and Research Innovation Grant for Graduate of Jiangsu Common High School (GXZZ13-0225).References
- V. M. Boddu, K. Abburi, A. J. Fredricksen, S. W. Maloney and R. Damavarapu, Environ. Technol., 2009, 30, 173–181 CrossRef CAS PubMed.
- W. E. Platten, D. Bailey, M. T. Suidan and S. W. Maloney, Chemosphere, 2010, 81, 1131–1136 CrossRef CAS PubMed.
- N. N. Perreault, D. Manno, A. Halasz, S. Thiboutot, G. Ampleman and J. Hawari, Biodegradation, 2012, 23, 287–295 CrossRef CAS PubMed.
- J. Y. Shen, C. J. Ou, Z. Y. Zhou, J. Chen, K. X. Fang, X. Y. Sun, J. S. Li, L. Zhou and L. J. Wang, J. Hazard. Mater., 2013, 260, 993–1000 CrossRef CAS PubMed.
- S. Susarla, Y. Yonezawa and S. Masunaga, Water Res., 1998, 32, 639–648 CrossRef CAS.
- B. A. Donlon, E. Razo-Flores, J. A. Field and G. Lettinga, Appl. Environ. Microbiol., 1995, 61, 3889–3893 CAS.
- J. Singh, S. Comfort and P. Shea, J. Environ. Qual., 1998, 27, 1240–1245 CrossRef CAS.
- B. T. Oh, C. L. Just and P. J. J. Alvarez, Environ. Sci. Technol., 2001, 35, 4341–4346 CrossRef CAS.
- C. B. Wang and W. X. Zhang, Environ. Sci. Technol., 1997, 31, 2154–2156 CrossRef CAS.
- L. M. Ma and W. X. Zhang, Environ. Sci. Technol., 2008, 42, 5384–5389 CrossRef CAS.
- S. C. Ahn, D. K. Cha, B. J. Kim and S.-Y. Oh, J. Hazard. Mater., 2011, 192, 909–914 CrossRef CAS PubMed.
- S. Y. Oh, P. C. Chiu, B. J. Kim and D. K. Cha, Water Res., 2005, 39, 5027–5032 CrossRef CAS PubMed.
- J. Shen, Z. Zhou, C. Ou, X. Sun, J. Li, W. Han, L. Zhou and L. Wang, J. Environ. Sci., 2012, 24, 1900–1907 CrossRef CAS.
- W. W. Li, Y. Zhang, J. B. Zhao, Y. L. Yang, R. J. Zeng, H. Q. Liu and Y. J. Feng, Bioresour. Technol., 2013, 149, 38–43 CrossRef CAS PubMed.
- Y. B. Zhang, Y. W. Liu, Y. W. Jing, Z. Q. Zhao and X. Quan, J. Environ. Sci., 2012, 24, 720–727 CrossRef CAS.
- X. Xiao, G. P. Sheng, Y. Mu and H. Q. Yu, Water Res., 2013, 47, 6007–6013 CrossRef CAS PubMed.
- W. Yin, J. Wu, P. Li, G. Lin, X. Wang, B. Zhu and B. Yang, Chem. Eng. J., 2012, 210, 309–315 CrossRef CAS PubMed.
- Y. Liu, Y. Zhang, Z. Zhao, Y. Li, X. Quan and S. Chen, Bioresour. Technol., 2012, 121, 148–153 CrossRef CAS PubMed.
- X. Y. Yu, C. Amrhein, M. A. Deshusses and M. R. Matsumoto, Environ. Sci. Technol., 2006, 40, 1328–1334 CrossRef CAS.
- J. Shen, R. He, H. Yu, L. Wang, J. Zhang, X. Sun, J. Li, W. Han and L. Xu, Bioresour. Technol., 2009, 100, 1922–1930 CrossRef CAS PubMed.
- C. Olivares, J. Liang, L. Abrell, R. Sierra-Alvarez and J. A. Field, Biotechnol. Bioeng., 2013, 110, 1595–1604 CrossRef CAS PubMed.
- J. Fan, Y. Guo, J. Wang and M. Fan, J. Hazard. Mater., 2009, 166, 904–910 CrossRef CAS PubMed.
- M. Hou, F. Li, X. Liu, X. Wang and H. Wan, J. Hazard. Mater., 2007, 145, 305–314 CrossRef CAS PubMed.
- L. Liang, W. Sun, X. Guan, Y. Huang, W. Choi, H. Bao, L. Li and Z. Jiang, Water Res., 2014, 49, 371–380 CrossRef CAS PubMed.
- L. Zhu, H. Lin, J. Qi and X. Xu, Environ. Sci. Pollut. Res., 2013, 20, 6119–6127 CrossRef CAS PubMed.
- J. Huang, Y. Wen, N. Ding, Y. Xu and Q. Zhou, Water Res., 2012, 46, 4361–4370 CrossRef CAS PubMed.
- V. Murali, S.-A. Ong, L.-N. Ho and Y. S. Wong, Bioresour. Technol., 2013, 143, 104–111 CrossRef CAS PubMed.
- Y. Liu, Y. Zhang, X. Quan, Y. Li, Z. Zhao, X. Meng and S. Chen, Chem. Eng. J., 2012, 192, 179–185 CrossRef CAS PubMed.
- X. Meng, Y. Zhang, Q. Li and X. Quan, Biochem. Eng. J., 2013, 73, 80–85 CrossRef CAS PubMed.
- X. Quan, X. Zhang and H. Xu, Water Res., 2015, 78, 74–83 CrossRef CAS PubMed.
- Z. Z. Ismail and S. G. Pavlostathis, Biodegradation, 2010, 21, 43–57 CrossRef CAS PubMed.
- C. F. Shen, N. Kosaric and R. Blaszczyk, Water Res., 1993, 27, 25–33 CrossRef CAS.
- G. Zhen, X. Lu, Y. Y. Li, Y. Liu and Y. Zhao, Chem. Eng. J., 2015, 263, 461–470 CrossRef CAS PubMed.
- S. Karri, R. Sierra-Alvarez and J. A. Field, Biotechnol. Bioeng., 2005, 92, 810–819 CrossRef CAS PubMed.
- D. O. Tas and S. G. Pavlostathis, Environ. Sci. Technol., 2005, 39, 8264–8272 CrossRef CAS.
- W. Zhang, L. Chen, H. Chen and S. Q. Xia, J. Hazard. Mater., 2007, 143, 57–64 CrossRef CAS PubMed.
- J. F. Devlin and K. O. Allin, Environ. Sci. Technol., 2005, 39, 1868–1874 CrossRef CAS.
- R. K. Thauer, K. Jungermann and K. Decker, Bacteriol. Rev., 1977, 41, 100–180 CAS.
- P. Clauwaert, K. Rabaey, P. Aelterman, L. de Schamphelaire, T. H. Ham, P. Boeckx, N. Boon and W. Verstraete, Environ. Sci. Technol., 2007, 41, 3354–3360 CrossRef CAS.
- M. Uchimiya, L. Gorb, O. Isayev, M. M. Qasim and J. Leszczynski, Environ. Pollut., 2010, 158, 3048–3053 CrossRef CAS PubMed.
- H. Hwang, D. G. Kim and H. S. Shin, J. Hazard. Mater., 2011, 185, 1513–1521 CrossRef PubMed.
- M. J. Alowitz and M. M. Scherer, Environ. Sci. Technol., 2002, 36, 299–306 CrossRef CAS.
- K. Ritter, M. S. Odziemkowski, R. Simpgraga, R. W. Gillham and D. E. Irish, J. Contam. Hydrol., 2003, 65, 121–136 CrossRef CAS.
- T. Suzuki, M. Moribe, Y. Oyama and M. Niinae, Chem. Eng. J., 2012, 183, 271–277 CrossRef CAS PubMed.
- Y. H. Huang and T. C. Zhang, Water Res., 2006, 40, 3075–3082 CrossRef CAS PubMed.
- F. P. van der Zee, I. A. E. Bisschops, V. Blanchard, R. H. M. Bouwman, G. Lettinga and J. A. Field, Water Res., 2003, 37, 3098–3109 CrossRef CAS.
- M. A. Reis, J. S. Almeida, P. C. Lemos and M. J. Carrondo, Biotechnol. Bioeng., 1992, 40, 593–600 CrossRef CAS PubMed.
- A. J. Pyzik and S. E. Sommer, Geochim. Cosmochim. Acta, 1981, 45, 687–698 CrossRef CAS.
- L. A. Hoferkamp and E. J. Weber, Environ. Sci. Technol., 2006, 40, 2206–2212 CrossRef CAS.
- M. Elsner, R. P. Schwarzenbach and S. B. Haderlein, Environ. Sci. Technol., 2004, 38, 799–807 CrossRef CAS.
- J. Hawari, F. Monteil-Rivera, N. N. Perreault, A. Halasz, L. Paquet, Z. Radovic-Hrapovic, S. Deschamps, S. Thiboutot and G. Ampleman, Chemosphere, 2015, 119, 16–23 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11197h |
| This journal is © The Royal Society of Chemistry 2015 |


