Magnesium hydroxide nanoplate/graphene oxide composites as efficient adsorbents for organic dyes†
Abstract
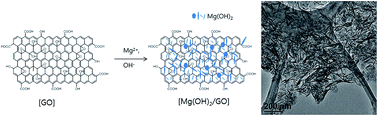
Nanocomposites comprised of magnesium hydroxide (Mg(OH)2) and graphene oxide (GO) were prepared by the controlled precipitation of a magnesium salt on the GO surface. The population of Mg(OH)2 nanocrystals on the GO surface could be varied by varying the Mg(OH)2 precursor amount; the surface area of the resulting Mg(OH)2/GO nanocomposites varied from 75.2 m2 g−1 to 465 m2 g−1. The Mg(OH)2/GO nanocomposite with a surface area of 465 m2 g−1 showed the best performance. Owing to the synergistic effect of the nanoplate structure of Mg(OH)2 and the 2D structure of GO, the obtained Mg(OH)2/GO nanocomposites exhibited high performance in methylene blue adsorption (adsorption capacity: 779.4 mg g−1).

 Please wait while we load your content...
Please wait while we load your content...