Ohmic heating assisted synthesis of coumarinyl porphyrin derivatives†
M. F. do C. Cardosoab,
A. T. P. C. Gomesa,
V. L. M. Silva*a,
A. M. S. Silvaa,
M. G. P. M. S. Nevesa,
F. de C. da Silvab,
V. F. Ferreira*b and
J. A. S. Cavaleiro*a
aDepartment of Chemistry and QOPNA, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: verasilva@ua.pt; jcavaleiro@ua.pt
bUniversidade Federal Fluminense, Instituto de Química, Departamento de Química Orgânica, Valonguinho, 24020-150, Niterói, RJ, Brazil. E-mail: cegvito@vm.uff.br
First published on 30th July 2015
Abstract
Ohmic heating offers a very efficient way of performing organic reactions using water as solvent. An efficient access to new coumarinyl porphyrin derivatives bearing pyrano[3,2-c]coumarin motifs at the β-pyrrolic position of porphyrin macrocycles is disclosed by using this type of heating. The synthetic strategy involved a sequential Knoevenagel and hetero-Diels–Alder reaction between 2-vinyl-5,10,15,20-tetraphenylporphyrinatozinc(II) and α-methylenechromane derivatives generated in situ from 4-hydroxycoumarin and aromatic aldehydes in aqueous media. The results obtained under ohmic heating were compared with those obtained under conventional heating using water and also organic solvents.
A. Introduction
One of the most important objectives in modern organic chemistry is the development of efficient synthetic routes leading to target compounds and whenever possible, under sustainable conditions fulfilling green chemistry requirements. The reactions should preferably be facile and fast, and the resulting products should be easily isolated and purified. To achieve such goals, alternative and more efficient heating processes are under consideration.Ohmic heating (ΩH) is defined as a process where an AC electric current is passed through a conductive medium (which behaves as an electrical resistor) with the primary purpose of heating it (Fig. 1). This process has been used in food processing1 but it can also be applied to heat chemical reactions, especially organic reactions performed in aqueous medium. It is an alternative to conventional heating and to microwave (MW) irradiation methods, as it has been demonstrated recently by Silva and coworkers.2 In ΩH, heat is internally generated due to electrical resistance. The dissipation of electrical energy into the reaction medium generates heat. Therefore, heating occurs in the form of internal energy transformation (from electric to thermal) within the reaction mixture. Thus, ΩH can be seen as an internal thermal energy generation technology, and not only as a thermal energy transfer. This means that it does not depend on the heat transfer to the medium, resulting in a high speed heating rate and thus giving rise to a rapid and uniform heating process leading to shorter reaction times and higher yields. (ΩH) differs from the other heating techniques by the presence of electrodes in contact with the reaction mixture allowing the use of a variable frequency and a variable waveform (Fig. 1); in general the sinusoidal waveform is selected.2
Both (MW) irradiation and (ΩH) have the ability to transform electrical energy into heat. However, (ΩH) allows a uniform heating, overcoming the problems associated with microwave heating, when large volumes are concerned, due to the low penetration depth of microwave radiation. Thus, the scaling of direct ohmic heating for the pilot or even the industrial scales should not present the limitations and difficulties found in microwave irradiation.
Combining the use of (ΩH) and water (as solvent) provides great opportunities for green chemistry approaches. Water has a high heat capacity and is non-toxic, non-flammable and easily available at low cost,3 and it is also known to enhance the rates and to affect the selectivity of a wide variety of organic reactions.4
To date four different types of organic reactions using (ΩH) were already performed and reported.2 Those include nucleophilic substitutions, amines N-alkylations, Suzuki cross-coupling and Diels–Alder reactions. In particular, the cycloaddition of 9-hydroxymethylanthracene with N-methylmaleimide has been reported to take place in a short reaction time (∼2 min.), giving a high yield of the cycloadduct, a much better procedure when compared with those using microwave and oil bath heating conditions.
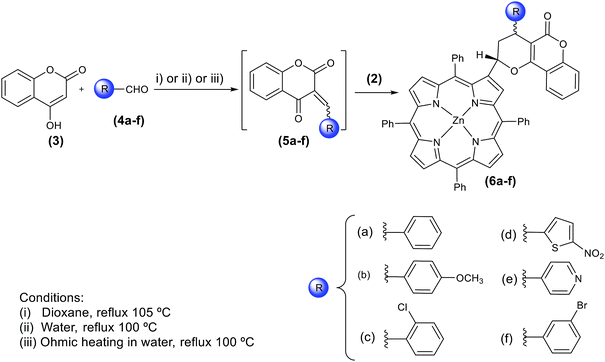
Herein, we have decided to evaluate the potentiality of (ΩH) to perform reactions with more complex systems such as those comprising tetrapyrrolic systems like porphyrins. The synthetic strategy selected involved a sequential Knoevenagel and hetero-Diels–Alder reactions of β-vinyl-meso-tetraphenyl porphyrinatozinc(II) with α-methylenechromane derivatives and these have been generated in situ from 4-hydroxycoumarin and a series of aromatic aldehydes (Scheme 1). The importance of such reactions is unquestionable since they constitute a new methodology for the synthesis of significant tetrapyrrolic macrocycles; the latter are of great interest due to their promising applications as catalysts in solar conversion,5 in biomimetic model systems of photosynthetic processes,6 as sensors and especially as photosensitizers in diagnosis and photodynamic therapy treatment of several types of cancer7 and for the photoinactivation of microorganisms.8
Important templates for further functionalisation are the 5,10,15,20-tetraphenylporphyrin 1 and the corresponding β-vinyl derivative 2. The structural modification leading to new derivatives of those porphyrinic macrocycle cores can be performed by incorporating various substituents and heterocyclic moieties at the porphyrin ring using, for instance, Diels–Alder and 1,3-dipolar cycloadditions, 1,5-electrocyclisations and cheletropic reactions.9 In order to obtain new compounds with these requirements it is necessary to study the development of novel synthetic methodologies leading to novel molecular frameworks from meso-tetraarylporphyrins.
In particular, the Aveiro group10 has been performing hetero-Diels–Alder reactions with porphyrins and analogues using extremely reactive dienes like ortho-quinone methides (o-QM). These reactive intermediates are usually generated and used in situ and a valuable method for their preparation is based on the Knoevenagel reaction of aldehydes with enols; o-QM can then be used in several reactions such as the hetero-Diels–Alder reactions.11 For instance, the later sequence has been successfully used in the derivatisation of the easily accessible 2-vinyl-5,10,15,20-tetraphenylporphyrinatozinc(II)12 leading to macrocycles containing 5,10-dioxo benzo[g]chromene, 5,6-dioxobenzo[h]chromene, pyrano[3,2-c]coumarin and benzopyran motifs at a porphyrin β-position. The synthesis of those compounds was performed using 1,4-dioxane and o-dichlorobenzene as solvents, and reaction times ranging from 1 to 48 hours and yields of 8–95%.10 In some cases, the addition of extra equivalents of the ortho-quinone methide was required.
So, as a further extension of our studies in this field, the present work reports the synthesis of coumarinyl porphyrin derivatives using (ΩH) in aqueous medium. The o-QM were obtained from 4-OH-coumarin and a series of aromatic aldehydes. The efficiency of these conditions was evaluated by comparing the new results with the ones obtained from reactions performed under conventional heating conditions and using water or dioxane12 as solvents.
B. Results and discussion
B.1. Chemistry
The experimental conditions explored in the synthesis of the new coumarinyl porphyrin derivatives 6a–f are outlined in Scheme 1 and the results obtained are summarised in Table 1. These reactions were performed by generating the adequate α-methylenechromane derivatives 5a–f in the presence of 2-vinyl-5,10,15,20-tetraphenylporphyrinatozinc(II)10 2. These highly reactive intermediates were obtained in situ from 4-OH-coumarin 3 and also from the corresponding aromatic aldehydes 4a–f; the reaction times required to obtain the desired coumarinyl porphyrins 6a–f and the yields of the two diastereomers (Scheme 2) were dependent on the reaction conditions.| Aldehydes | Products | Yields and reaction times | ||
|---|---|---|---|---|
| Cond. (i) | Cond. (ii) | Cond. (iii) | ||
| 4a | 6a1 | 85% (1 h) | 78% (24 h) | 92% (30 min) |
| 6a2 | 15% (1 h) | 20% (24 h) | 7% (30 min) | |
| 4b | 6b1 | 66% (24 h) | 56% (24 h) | 83% (1 h) |
| 6b2 | 30% (24 h) | 38% (24 h) | 16% (1 h) | |
| 4c | 6c1 | 80% (24 h) | 62% (24 h) | 90% (1 h) |
| 6c2 | 18% (24 h) | 27% (24 h) | 7% (1 h) | |
| 4d | 6d1 | 78% (24 h) | 62% (24 h) | 85% (1 h) |
| 6d2 | 20% (24 h) | 26% (24 h) | 14% (1 h) | |
| 4e | 6e1 | 77% (24 h) | 54% (24 h) | 88% (1 h) |
| 6e2 | 5% (24 h) | 11% (24 h) | 6% (1 h) | |
| 4f | 6f1 | 81% (24 h) | 68% (24 h) | 90% (1 h) |
| 6f2 | 12% (24 h) | 25% (24 h) | 5% (1 h) | |
Under conditions (i) (Scheme 1), the reaction with benzaldehyde afforded 6a (as two diastereomers 6a1 and 6a2) after 1 h under reflux in the presence of 1.0 equivalent of 4-OH-coumarin and 8.0 equivalents of the aldehyde, with total consumption of the starting porphyrin. With the other substituted aldehydes 4b–f it was required longer reaction times for an efficient consumption of the starting porphyrin; after 12 h of reaction it was added more aldehyde and coumarin in the same proportion. After 24 h of reaction, TLC of the reaction mixture showed the presence of two major compounds accompanied by a minor amount of the starting porphyrin. The correct assignment of the two new diastereomers in each case (Scheme 2) was based on the fully analysis of their 1H, 13C, COSY, HMBC, HSQC and NOESY NMR spectra, after purification by column and preparative thin-layer chromatography.
In particular, considering the diastereomers 6a, the expected addition of the vinylporphyrin 2 at the 3′,4′ positions of the α-methylenechromane derivatives10 was confirmed by the presence of a signal at δ 162.4 ppm in their 13C NMR spectra due to the carbon carbonyl C-5′ that is correlated with the hydrogen H-4′ (δ 3.82 ppm for 6a1 and δ 4.33 ppm for 6a2) in the HMBC spectrum. In addition it was also possible to confirm in the HMBC spectra the correlation of hydrogen H-4′ with C-4a′ (δ 104.7/101.9 ppm), C-10b′ (δ 161.4/161.3 ppm), C-2′ (δ 76.1/69.2 ppm) and C-3′ (δ 42.3/36.4 ppm).
From the 1H NMR spectra and mainly from the coupling constant values it is possible to differentiate both isomers. For instance in the case of diastereomers 6a: (a) in 6a1 both H-3′ appear at 2.42 (dt, J 14.5, 11.0 Hz) and 2.94 (ddd, J 14.5, 6.3, 1.5 Hz), while H-4′ (3.82, dd, J 11.0, 6.3 Hz) and H-2′ (5.26, br d, J 11.0 Hz) are more deshielded; and (ii) in 6a2 the resonances of one H-3′ and both H-4′ and H-5′ are more deshielded than the corresponding protons from the isomer 6a1 [2.42 (dd, J 14.3, 11.7 Hz, 1H, H-3′), 3.15 (m, 1H, H-3′), 4.33 (d, J 5.1 Hz, 1H, H-4′), 5.47 (dd, J 11.7, 1.3 Hz, 1H, H-2′)]. The NOESY spectrum was particularly important for the identification of the major product as 6a1 and the minor one as 6a2. In these spectra it was possible to see clearly that in 6a1 there is a correlation between the hydrogens H-2′ and H-4′, indicating that both hydrogens are spatially close. In the compound 6a2 such correlation was not observed. The same pattern of 1H, 13C and NOESY NMR spectra was also observed for the other pairs of diastereomers.
In the reactions performed in water under conventional heating (conditions (ii), Scheme 1) and under ohmic heating (conditions (iii), Scheme 1) the only products obtained were also the diastereomers 6a1–6f1 and 6a2–6f2. However, in water and under conventional heating all the aldehydes required 24 h for an efficient consumption of the starting porphyrin and it was necessary to add another proportion of coumarin and of the aldehyde after 12 h reaction time. Comparing the yields of isomers 6a1–6f1 and 6a2–6f2 under conventional heating in dioxane and water it was observed that the yields of the obtained isomers 6a1–6f1 decrease in this last solvent and those of isomers 6a2–6f2 increase.
The results obtained when using aqueous NaCl solution under ohmic heating are particularly gratifying. Excellent conversions were obtained after 30 min and 1 h of reaction and the main compounds 6a1–6f1 were obtained in higher yields when compared with the corresponding isomers 6a2–6f2 (Table 1). This result shows that when using (ΩH) and water as solvent these reactions are highly regioselective and site-selective.13 The use of the ohmic reactor to heat this reaction caused a significant improvement in the reaction efficiency, leading to a decrease of the reaction time and to an atom economy. This is contrary to what is observed under conditions (i) and (ii), where a second addition of both reagents, aldehyde and coumarin, was necessary.
Based on these results a question can be put forward: are there any non-thermal effects, for instance “electrochemical effects” in ohmic heating that could explain the differences in the reaction time, yields and selectivity found when compared with conventional heating? In a recent publication,14 Silva and co-workers suggested the existence of some “electrochemical effects” in the ohmic heating assisted Suzuki–Miyaura reaction of 3-iodo-1-methylquinolin-4(1H)-ones with arylboronic acids, that may be related to the enhancement of Pd catalyst deposition on the electrodes and its solubilisation in the reaction medium. The authors claimed that the high heating rates achieved in ohmic heating in the beginning of the reaction might enhance the reduction of Pd(II) to Pd(0) which is the species involved in the catalytic cycle of the Suzuki reaction. Moreover they suspected that the used iron-containing electrodes might reduce aqueous solutions of Pd(OAc)2 to the pure metal Pd(0). The deposition mechanism under the ohmic heating process itself may enforce this phenomenon. However ohmic heating in the context of organic synthesis is a new concept and as far as we know there are only three publications on this issue.2,14 Presently there are not sufficient data to rationalise about the existence of non-thermal or any specific effects of ohmic heating. What we can rationalise at this moment is that the high heating rates achieved in ohmic heating at the beginning of the reaction may lead to a more uniform heating and to less decomposition of the reactants. In addition, the electrical dynamic perturbation in ohmic heating may have a role in the improvement of the transport properties and polarization of the reaction medium. The metallic (stainless steel type 316) electrodes themselves may have a role in this sequential Knoevenagel and hetero-Diels–Alder reaction.15 Moreover the addition of NaCl to increase the medium conductivity and the initial heating rate may also have an effect in the reaction rate and selectivity. It is well known that salts with small cations are known to enhance the hydrophobic effect (salting-out effect) that could facilitate the Diels–Alder reactions in water.16 Despite the low concentration of the salt aqueous solution (∼0.028 M) when compared to those described in the literature16 we cannot exclude this effect. However additional research is necessary in order to investigate the thermal behaviour during heating and to fully understand the phenomena involved in the ohmic heating process.
B.2. General procedures
Analytical grade solvents were used. Reagents were purchased from Aldrich or Acros Chemicals. Column chromatography was performed on silica gel 60 (Merck 70–230 mesh). Yields refer to purified compounds obtained by chromatography techniques and confirmed by spectroscopic data. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.25 mm Merck silica gel plates (60 F-254) using UV light as visualising agent. NMR spectra were recorded on a Bruker Avance NMR equipment operating at 300 MHz for 1H and 75 MHz for 13C in DMSO-d6 and CDCl3 solutions and TMS was used as the internal standard (δ 0 ppm). In other cases NMR spectra were recorded on a Bruker Avance instrument operating at 500 MHz for 1H and 125 MHz for 13C in DMSO-d6 or CDCl3 solutions. The coupling constants (J) are reported in Hz and refer to apparent peak multiplicities.For experiments carried out under ohmic heating, the 10 mL reactor was filled with the reaction mixture, closed and the mixture was heated to reflux. To increase the conductivity NaCl (5 mg) was added to the reaction media. A pair of stainless steel type 316 electrodes arranged in parallel rods (4 mm of diameter) was immersed in the reaction medium. For 3–4 mL of reaction mixture the length of electrodes immersed in the reaction medium was 5–9 mm and the distance between the electrodes was 10 mm. The temperature measurement was done using a type J glass sheathed thermocouple located inside the reactor. All the experiments were carried out under medium magnetic stirring speed (740 rpm).
In an ohmic heating micro-reactor, equipped with a magnetic stirring bar, 4-hydroxycoumarin (3, 2.3 mg, 0.014 mmol), adequate aldehyde (0.114 mmol), porphyrin 2 (10 mg, 0.014 mmol) and NaCl (5 mg) in distilled water (3 mL) were heated at reflux until consumption of the starting porphyrin 2 (30 min to 1 h). After that period, CHCl3 (30 mL) was added to the reaction mixture and it was washed with saturated aqueous NaHCO3 (2 × 30 mL). The organic phase after being dried with anhydrous sodium sulphate was concentrated under vacuum, and the residual crude product was purified by column chromatography on silica gel and subsequently by preparative TLC using CHCl3 as the eluent.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 425 (4.91), 553 (4.55), 595 (4.00) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H40N4O3Zn: 953.2392; found: 953.2394.
ε): 425 (4.91), 553 (4.55), 595 (4.00) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H40N4O3Zn: 953.2392; found: 953.2394.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 425 (4.90), 552 (4.55), 596 (4.00) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H40N4O3Zn: 953.2392; found: 953.2393.
ε): 425 (4.90), 552 (4.55), 596 (4.00) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H40N4O3Zn: 953.2392; found: 953.2393.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 424 (4.93), 555 (4.57), 595 (4.55) nm. HRMS (ESI+): m/z [M + H]+ calcd for C63H42N4O4Zn: 983.2498; found: 983.2495.
ε): 424 (4.93), 555 (4.57), 595 (4.55) nm. HRMS (ESI+): m/z [M + H]+ calcd for C63H42N4O4Zn: 983.2498; found: 983.2495.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 423 (4.93), 554 (4.58), 595 (4.55) nm. HRMS (ESI+): m/z [M + H]+ calcd for C63H42N4O4Zn: 983.2498; found: 983.2497.
ε): 423 (4.93), 554 (4.58), 595 (4.55) nm. HRMS (ESI+): m/z [M + H]+ calcd for C63H42N4O4Zn: 983.2498; found: 983.2497.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 422 (5.75), 548 (4.60), 593 (4.51) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H39ClN4O3Zn: 987.2002; found: 987.2006.
ε): 422 (5.75), 548 (4.60), 593 (4.51) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H39ClN4O3Zn: 987.2002; found: 987.2006.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 421 (5.73), 547 (4.60), 591 (4.50) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H39ClN4O3Zn: 987.2002; found: 987.2004.
ε): 421 (5.73), 547 (4.60), 591 (4.50) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H39ClN4O3Zn: 987.2002; found: 987.2004.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 420 (5.72), 547 (4.59), 596 (4.50) nm. HRMS (ESI+): m/z [M + H]+ calcd for C60H37N5O5SZn: 1004.1807; found: 1004.1801.
ε): 420 (5.72), 547 (4.59), 596 (4.50) nm. HRMS (ESI+): m/z [M + H]+ calcd for C60H37N5O5SZn: 1004.1807; found: 1004.1801.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 422 (5.71), 547 (4.60), 595 (4.50) nm. HRMS (ESI+): m/z [M + H]+ calcd for C60H37N5O5SZn: 1004.1807; found: 1004.1804.
ε): 422 (5.71), 547 (4.60), 595 (4.50) nm. HRMS (ESI+): m/z [M + H]+ calcd for C60H37N5O5SZn: 1004.1807; found: 1004.1804.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 422 (4.95), 544 (4.56) nm. HRMS (ESI+): m/z [M + H]+ calcd for C61H39N5O3Zn: 954.2344; found: 954.2349.
ε): 422 (4.95), 544 (4.56) nm. HRMS (ESI+): m/z [M + H]+ calcd for C61H39N5O3Zn: 954.2344; found: 954.2349.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 423 (4.95), 545 (4.55) nm. HRMS (ESI+): m/z [M + H]+ calcd for C61H39N5O3Zn: 954.2344; found: 954.2345.
ε): 423 (4.95), 545 (4.55) nm. HRMS (ESI+): m/z [M + H]+ calcd for C61H39N5O3Zn: 954.2344; found: 954.2345.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 420 (4.98), 544 (4.58) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H39BrN4O3Zn: 1031.1497; found: 1031.1494.
ε): 420 (4.98), 544 (4.58) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H39BrN4O3Zn: 1031.1497; found: 1031.1494.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 420 (4.97), 546 (4.58) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H39BrN4O3Zn: 1031.1497; found: 1031.1490.
ε): 420 (4.97), 546 (4.58) nm. HRMS (ESI+): m/z [M + H]+ calcd for C62H39BrN4O3Zn: 1031.1497; found: 1031.1490.C. Conclusions
This study has shown that complex reactions such as the sequence of Knoevenagel and hetero-Diels–Alder reactions of 2-vinyl-5,10,15,20-tetraphenylporphyrinato zinc(II) (2) with α-methylenechromane derivatives obtained from hydroxycoumarin and aldehydes can be successfully performed using the ohmic heating reactor, leading to macrocycles containing the coumarin moiety at the β-pyrrolic positions. Six new coumarinyl porphyrin derivatives and their respective diastereoisomers were isolated. When compared to the conventional heating, the use of ohmic heating in these reactions led to a significant reduction of the reaction time, cleaner reaction mixtures and higher yields and selectivities. Moreover, atom economy was observed for the reactions carried out in the ohmic heating reactor since a second addition of aldehyde and coumarin was not necessary under these conditions. Furthermore, the use of water or of an aqueous solution as solvent facilitates the workup and product isolation over traditional solvents.Acknowledgements
Thanks are due to FCT/MEC for the financial support to the QOPNA research Unit (FCT UID/QUI/00062/2013), through national funds and where applicable to those co-financed by the FEDER, within the PT2020 Partnership Agreement, and also to the Portuguese NMR Network. V. L. M. Silva thanks project New Strategies Applied to Neuropathological Disorders (CENTRO-07-ST24-FEDER-002034), co-funded by QREN, “Mais Centro-Programa Operacional Regional do Centro” and EU, and FEDER for her Assistant Research position; A. T. P. C. Gomes thanks FCT for her grant (SFRH/BPD/79521/2011). Thanks are also due to the CAPES (Brazil)-FCT (Portugal) agreement for funding. M. F. C. Cardoso thanks CAPES for her grant related with her research stay in Aveiro.Notes and references
- M. C. Knirscha, C. A. Santos, A. A. M. O. S. Vicente and T. C. V. Penna, Trends Food Sci. Technol., 2010, 20, 1 Search PubMed.
- (a) J. Pinto, V. L. M. Silva, A. M. G. Silva, A. M. S. Silva, J. C. S. Costa, L. M. N. B. F. Santos, R. Enes, J. A. S. Cavaleiro, A. A. M. O. S. Vicente and J. A. C. Teixeira, Green Chem., 2013, 15, 970 RSC; (b) V. L. M. Silva, A. M. S. Silva, L. M. N. B. F. Santos, A. M. G. Silva, J. Pinto, R. Enes, J. A. S. Cavaleiro, A. A. M. O. S. Vicente, J. A. C. Teixeira, A. Morais and J. C. S. Costa, Portuguese Patent, 105908, 2011-09-27.
- (a) T. Head-Gordon and G. Hura, Chem. Rev., 2002, 102, 2651 CrossRef CAS PubMed; (b) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 CrossRef CAS PubMed; (c) F. G. Moore and G. L. Richmond, Acc. Chem. Res., 2008, 41, 739 CrossRef CAS PubMed; (d) Y. R. Shen and V. Ostroverkhov, Chem. Rev., 2006, 106, 1140 CrossRef CAS PubMed.
- (a) C.-J. Li and T.-H. Chan, Organic reactions in aqueous media, Wiley, New York, 1997 Search PubMed; (b) Organic Synthesis in Water, ed. P. A. Grieco, Blackie, London, 1998 Search PubMed.
- (a) J. R. Darwent, P. Douglas, A. Harriman, G. Porter and M. C. Richoux, Coord. Chem. Rev., 1982, 44, 83 CrossRef CAS; (b) A. Yella, H. W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W. G. Diau, C. Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef CAS PubMed.
- (a) L. R. Milgrom, The Colours of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds, Oxford University Press, Oxford, 1997 Search PubMed; (b) N. M. M. Moura, C. Núñez, M. A. F. Faustino, J. A. S. Cavaleiro, M. G. P. M. S. Neves, J. L. Capelo and C. Lodeiro, J. Mater. Chem. C, 2014, 2, 4772 RSC; (c) N. M. M. Moura, C. Núñez, S. M. Santos, M. A. F. Faustino, J. A. S. Cavaleiro, M. G. P. M. S. Neves, J. L. Capelo and C. Lodeiro, Chem.–Eur. J., 2014, 20, 6684 CrossRef CAS PubMed.
- (a) T. J. Dougherty, Photochem. Photobiol., 1987, 45, 879 CrossRef CAS PubMed; (b) K. M. Kadish, K. M. Smith and R. Guilard, The Porphyrin Handbook, Academic Press, Boston, 2000, vol. 6, p. 45 Search PubMed; (c) K. Berg, P. K. Selbo, A. Weyergang, A. Dietze, L. Prasmickaite, A. Bronsted, B. Engesaeter, E. Angellpetersen, T. Warloe, N. Frandsen and A. Høgset, J. Microsc., 2005, 218, 133 CrossRef CAS PubMed; (d) S. Pervaiz and M. Olivo, Clin. Exp. Pharmacol. Physiol., 2006, 33, 551 CrossRef CAS PubMed; (e) A. T. P. C. Gomes, M. A. F. Faustino, M. G. P. M. S. Neves, V. F. Ferreira, A. Juarranz, J. A. S. Cavaleiro and F. Sanz-Rodríguez, RSC Adv., 2015, 5, 33496 RSC.
- (a) E. Alves, L. Costa, C. M. B. Carvalho, J. P. C. Tomé, M. A. Faustino, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, A. Cunha and A. Almeida, BMC Microbiol., 2009, 9, 70 CrossRef PubMed; (b) E. Alves, M. A. F. Faustino, M. G. P. M. S. Neves, Â. Cunha, H. Nadais and A. Almeida, J. Photochem. Photobiol., C, 2015, 22, 34 CrossRef CAS PubMed; (c) J. Almeida, J. P. C. Tomé, M. G. P. M. S. Neves, A. C. Tomé, J. A. S. Cavaleiro, A. Cunha, M. A. F. Faustino and A. Almeida, Photochem. Photobiol. Sci., 2014, 13, 626 RSC.
- (a) J. A. S. Cavaleiro, M. G. P. M. S. Neves and A. C. Tomé, ARKIVOC, 2003, xiv, 107 CrossRef; (b) J. A. S. Cavaleiro, A. C. Tomé and M. G. P. M. S. Neves, meso-Tetraarylporphyrin derivatives: new synthetic methodologies, Handbook of Porphyrin Science, ed. K. M. Kadish, K. M. Smith and R. Guilard, World Scientific, Singapore, 2010, vol. 2, p. 193 Search PubMed; (c) N. M. M. Moura, M. A. F. Faustino, M. G. P. M. S. Neves, A. C. Tomé, E. M. Rakib, A. Hannioui, S. Mojhadi, S. Hackbarth, B. Roder, F. A. A. Paz, A. M. S. Silva and J. A. S. Cavaleiro, Tetrahedron, 2012, 68, 8181 CrossRef CAS PubMed.
- J. M. D. S. Menezes, A. T. P. C. Gomes, A. M. S. Silva, M. A. F. Faustino, M. G. P. M. S. Neves, A. C. Tomé, F. C. da Silva, V. F. Ferreira and J. A. S. Cavaleiro, Synlett, 2011, 13, 1841 Search PubMed.
- (a) S. E. Rokita, Quinone Methides, Wiley, Hoboken, 2009, pp. 1–25 Search PubMed; (b) L. F. Tietze and N. Rackelman, in, Multicomponent Reactions, ed. J. Zhu and H. Bienaymé, Wiley-VCH, Weinheim, 2005, p. 121 Search PubMed; (c) F. C. da Silva, S. B. Ferreira, C. R. Kaiser, A. C. Pinto and V. F. Ferreira, J. Braz. Chem. Soc., 2009, 20, 1478 CrossRef CAS; (d) S. B. Ferreira, F. C. da Silva, F. A. F. M. Bezerra, M. C. S. Lourenço, C. R. Kaiser, A. C. Pinto and V. F. Ferreira, Arch. Pharm. Chem. Life Sci., 2010, 343, 81 CAS; (e) G. Appendino, G. Cravotto, L. Toma, R. Annunziata and G. Palmisano, J. Org. Chem., 1994, 59, 5556 CrossRef CAS; (f) M. J. Khoshkholgh, M. Lotfi, S. Balalaie and F. Rominger, Tetrahedron, 2009, 65, 4228 CrossRef CAS PubMed; (g) J. S. Yadav, B. V. S. Reddy, D. Narsimhaswamy, P. N. Lakshmi, K. Narsimulu, G. Srinivasulu and A. C. Kunwar, Tetrahedron Lett., 2004, 45, 3493 CrossRef CAS PubMed.
- H. J. Callot, Tetrahedron, 1973, 29, 899 CrossRef CAS.
- H. Wang, Y. Wang, K. L. Han and X. J. Peng, J. Org. Chem., 2005, 70, 4910 CrossRef CAS PubMed.
- J. Pinto, V. L. M. Silva, A. M. G. Silva, L. M. N. B. F. Santos and A. M. S. Silva, J. Org. Chem., 2015, 80, 6649 CrossRef CAS PubMed.
- E. M. Schneider, M. Zeltner, N. Kränzlin, R. N. Grassa and W. J. Stark, Chem. Commun., 2015, 51, 10695 RSC; A. Kumar, M. Dewan, A. Saxena, A. De and S. Mozumdar, Catal. Commun., 2010, 11, 679 CrossRef CAS PubMed.
- (a) A. Kumar, Chem. Rev., 2001, 101, 1 CrossRef CAS PubMed; (b) A. Kumar and S. S. Pawar, Tetrahedron, 2002, 58, 1745 CrossRef CAS; (c) G. Graziano, J. Phys. Org. Chem., 2004, 17, 100 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Molecular synthesis and structure characterization data. See DOI: 10.1039/c5ra11156k |
| This journal is © The Royal Society of Chemistry 2015 |