Study on the efficiency of some amine derivatives as corrosion and scale inhibitors in cooling water systems†
M. A. Migahed*a,
A. A. Attiab and
R. E. Habibb
aEgyptian Petroleum Research Institute, Nasr City, Cairo, 11727, Egypt. E-mail: mohamedatiyya707@hotmail.com
bChemistry Department, Faculty of Science, Zagazig University, Egypt
First published on 17th June 2015
Abstract
Corrosion and scaling are the main problems in cooling water systems. To address these problems, this paper aimed to study the inhibition efficiency of some amine derivatives for carbon steel in sea water using electrochemical techniques such as potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). The selected compounds, with different structures and sizes, were found to be adsorbed onto the carbon steel surface and had good performance as corrosion and scale inhibitors. Potentiodynamic polarization studies indicate that the amine derivatives act as mixed type corrosion inhibitors, while the data obtained from EIS revealed that the value of the charge transfer resistance (Rt) increased when increasing the inhibitor concentration leading to an increase in the inhibition efficiency. Also, the obtained results from this study show that the selected amines can decrease scale build-up growth under the experimental conditions. The surface morphology of the carbon steel was investigated in the absence and presence of the selected inhibitors through scanning electron microscopy (SEM) and energy dispersive analysis of X-rays.
1. Introduction
Two of the main problems in cooling water systems are corrosion and scale phenomena. These problems have a great economic impact, since the first involves deterioration of the metallic surface, whereas the second causes loss of capacity for thermal exchange. These phenomena occur simultaneously in many industrial applications like cooling systems, and hence a study of their development and inhibition under real conditions becomes an issue of great interest. Significant scientific and technological efforts have been made to control these phenomena, yet they are still controlled through the addition of chemicals that inhibit their development. Many inhibitors have been used in cooling water systems in order to solve these problems.1–4 Corrosion and scale occur because of the electrochemical oxidation reduction reaction and the metal salt sediments on the metal surface.5Water is the most commonly used cooling fluid in industrial systems, such as heat exchangers, cooling towers, and related equipment. Depending on the quality and availability of a fresh water supply, the recirculating cooling water systems contain varying amounts of solids, suspended or dissolved, but most likely both.6 Dissolved solids usually consist of mineral complexes in the form of crystals, which, in a process described as nucleation, continue to grow. They are characterized by the extent of their solubility and especially by their potential insolubility at particular temperatures.
Carbon steel has many industrial applications because of its easy availability, low cost and uncomplicated fabrication, and it is used extensively in water pipe lines,7,8 cooling water systems,9 boilers, process industries, oil and gas, refining and extraction, etc.
Corrosion occurs when an electric current flows from one part of the metal (anode) through the water (electrolyte) to another part of the metal (cathode). Corrosion takes place at the anode only. This process degrades the metal, reduces its strength and thickness, and in some extreme cases, creates pits and then holes in the material. Corrosion, in general, and pitting corrosion, in particular, must be guarded against in order to ensure the long term integrity of a cooling system.10
Corrosion inhibitors are substances which, when added in small concentrations to a corrosive media, decrease or prevent the reaction of the metal with the media.11 Most of the effective inhibitors are molecules that contain heteroatoms such as O, N, and S and multiple bonds through which they are adsorbed onto the metal surface.12–21 It has been observed that adsorption depends mainly on certain physicochemical properties of the inhibitor group, such as the functional groups, electron density at the donor atoms, p-orbital character, and electronic structure of the molecule.22–47 Amines are well known as corrosion inhibitors of iron and its alloys. The relatively high water solubility of low molecular weight amines is an advantage for their use as corrosion inhibitors.48–53 The efficiency of an organic compound as an inhibitor is mainly dependent on its ability to be adsorbed onto the metal surface, which involves the replacement of water molecules at the corroding interface.
Scale is formed from minerals, formerly dissolved in water, that were deposited from water onto heat transfer surfaces or in-flow water lines. As water is evaporated in a cooling tower, the concentration of dissolved solids becomes greater until the solubility of a particular scale-causing mineral salt is exceeded. The most common scaling minerals are calcium carbonate (CaCO3), calcium phosphate Ca3(PO4)2, calcium sulfate (CaSO4), and silica. Formation of magnesium silicate scale is also possible under certain conditions. Silica will form in areas with the lowest water temperature, such as in the cooling tower fill.54 The critical parameters for cooling water are: conductivity, total dissolved solids (TDS), hardness, pH, alkalinity and saturation index.55 A number of techniques, such as the controlled acidification of sea water,56 use of anti-scale agents57,58 and utilization of sponge–ball cleaning, are employed to control scaling.
Some amine derivatives have been evaluated as corrosion inhibitors against several industrial media,59 and they showed inhibition efficiencies in the range of 50–80%. Since scale formation and metal corrosion problems appear in cooling water systems, this work aimed to examine the inhibition efficiency of some amine derivatives as corrosion and scale inhibitors for cooling water systems. The added value of this work arises from the point that the prepared compounds act as both corrosion and scale inhibitors which provides double action protective properties. Furthermore, using a double action chemical to replace the injection of two different chemicals into cooling water systems provides an economic advantage. Moreover, it eliminates the potential interference in the case of using two different chemical injections for corrosion and scale inhibition in a system. This work is the onset of a series of works currently under investigation in our labs.
2. Experimental
2.1. Materials
2.1.1.1. Chemical composition of the tested carbon steel. The average range of the chemical components of the tested carbon steel are shown in Table 1.
| Element | C | Si | Mn | P | S | Ni | Cr | Mo | V | Cu | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 0.09 | 0.22 | 1.52 | 0.01 | 0.05 | 0.04 | 0.02 | 0.004 | 0.002 | 0.02 | 0.04 | Rest |
| Species | Concentration | |
|---|---|---|
| mmol−1 kg−1 | g kg−1 | |
| Na+ | 468.5 | 10.77 |
| K+ | 10.21 | 0.399 |
| Mg2+ | 53.08 | 1.290 |
| Ca2+ | 10.28 | 0.4121 |
| Sr2+ | 0.090 | 0.0079 |
| Cl− | 545.9 | 19.354 |
| Br− | 0.842 | 0.0673 |
| F− | 0.068 | 0.0013 |
| HCO3− | 2.30 | 0.140 |
| SO42− | 28.23 | 2.712 |
| B(OH)3 | 0.416 | 0.0257 |
The most important properties of sea water are:
(i) Remarkably constant ratio of the concentrations of the major constituents worldwide.
(ii) High salt concentration, mainly sodium chloride.
(iii) High electrical conductivity.
(iv) Relatively high and constant pH.
(v) Buffering capacity.
(vi) Solubility of gases, of which oxygen and carbon dioxide in particular are of importance in the context of corrosion.
(vii) The presence of a myriad of organic compounds.
(viii) The existence of biological life, to be further distinguished as microfouling (e.g., bacteria, slime) and macrofouling (e.g., seaweed, mussels, barnacles, and many kinds of animals or fish).
Some of these factors are interrelated and depend on physical, chemical, and biological variables, such as depth, temperature, intensity of light, and the availability of nutrients. The main numerical specification of sea water is its salinity.
2.2. Inhibitors
Amines and their derivatives are good selected compounds in this work which inhibit the corrosion and scale deposition of carbon steel in cooling water systems. The chemical structure, 3D structure, nomenclature and molecular weight of the inhibitors used in this work are listed in Table 3.2.3. Techniques
3. Results and discussion
3.1. Potentiodynamic polarization measurements
Dynamic potential polarization is a common method that evaluates the inhibition efficiency of corrosion inhibitors.61The electrochemical measurements were carried out in a cell using a three electrode mode; a platinum electrode and saturated calomel electrode (SCE) were used as the counter and reference electrodes respectively. A 1 cm2 area of the carbon steel sample as the working electrode (WE) was abraded, washed, and finally immersed in the cooling water. The working electrode was first immersed into the test solution for 1 h to establish a steady state open circuit potential. After determining the open circuit potential, potentiodynamic polarization curves were obtained with a scan rate of 1 mV s−1 in both cathodic and anodic potentials to investigate the polarization behavior. Fig. 1 shows the cathodic and anodic polarization curves of carbon steel immersed in sea water in the absence and presence of different concentrations of compound III as a representative sample.
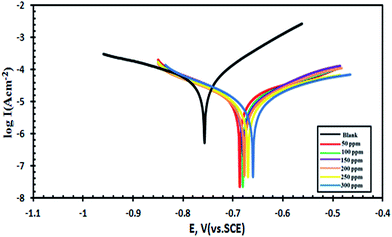 | ||
Fig. 1 Potentiodynamic polarization curves (E–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I relationship) of carbon steel in sea water in the absence and presence of different concentrations of compound III. I relationship) of carbon steel in sea water in the absence and presence of different concentrations of compound III. | ||
Electrochemical parameters such as the corrosion potential (Ecorr), corrosion current density (Icorr), and cathodic and anodic Tafel slopes (βc and βa) were calculated62 and listed in Table 4. The inhibition efficiency (IE%) values were evaluated from the measured Icorr values using the following equation:
| IE% = [(Icorr − Icorr(inh)/Icorr)] × 100 | (1) |
| Inhibitor | Conc., ppm | −Ecorr, mV vs. SCE | Icorr, μA cm−2 | βa, mV dec−1 | βc, mV dec−1 | IE% |
|---|---|---|---|---|---|---|
| Blank | 0 | 760 | 25.62 (±1.39) | 119.1 | 301.4 | — |
| (I) | 50 | 776.1 | 14.14 (±0.65) | 115.1 | 229.6 | 44.78 (±1.99) |
| 100 | 738 | 12.28 (±0.65) | 112.3 | 149.7 | 51.89 (±4.92) | |
| 150 | 807.6 | 10.97 (±0.65) | 133.2 | 206.8 | 57.18 (±0.34) | |
| 200 | 737.1 | 10.51 (±0.86) | 129.6 | 162.8 | 59.01 (±1.69) | |
| 250 | 734.4 | 9.15 (±0.74) | 125.2 | 138.4 | 64.31 (±1.54) | |
| 300 | 699.8 | 6.44 (±0.83) | 127.7 | 157.2 | 74.93 (±2.00) | |
| (II) | 50 | 788.6 | 13.02 (±1.16) | 125.2 | 211.7 | 49.23 (±2.68) |
| 100 | 735.7 | 11.96 (±0.60) | 120.9 | 190.8 | 53.30 (±1.13) | |
| 150 | 796.4 | 10.76 (±0.95) | 135.4 | 216.7 | 57.98 (±3.44) | |
| 200 | 714.1 | 9.20 (±0.60) | 135.2 | 195.3 | 64.09 (±1.43) | |
| 250 | 695.5 | 7.99 (±0.63) | 130.7 | 188.1 | 68.80 (±2.03) | |
| 300 | 651.7 | 5.68 (±0.85) | 131.6 | 144.5 | 77.83 (±2.76) | |
| (III) | 50 | 689.1 | 9.01 (±0.72) | 247.8 | 168.7 | 64.86 (±1.76) |
| 100 | 682.9 | 7.59 (±0.48) | 206.5 | 173.2 | 70.30 (±2.67) | |
| 150 | 682.1 | 6.70 (±0.53) | 199.4 | 171.3 | 73.87 (±0.88) | |
| 200 | 679.9 | 5.04 (±0.66) | 187.5 | 171.2 | 80.34 (±2.21) | |
| 250 | 672.6 | 3.85 (±0.58) | 195.1 | 160.7 | 84.99 (±1.89) | |
| 300 | 610.8 | 2.02 (±0.19) | 192.6 | 166.1 | 92.13 (±0.34) |
From Fig. 1, it is clear that both the anodic metal dissolution and cathodic reduction reactions were inhibited when compound III was added to the sea water and this inhibition was more pronounced with increasing inhibitor concentration. The Tafel lines were shifted from more negative to more positive potentials with respect to the blank curve by increasing the concentration of the inhibitor. The results show that an increase in the inhibitor concentration leads to a decrease in the corrosion current density (Icorr), but the βc and βa are approximately variable indicating that the retardation of the two reactions (cathodic oxygen reduction and anodic metal dissolution) was affected without changing the dissolution mechanism. The obtained results indicate that the percentage inhibition efficiency (IE%) of compound III is greater than that of compounds II and I.
3.2. Electrochemical impedance spectroscopy (EIS)
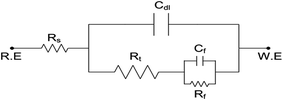
In order to understand the corrosion behavior of carbon steel in sea water in the absence and presence of various concentrations of compounds I, II and III, using compound III as a representative sample, electrochemical impedance spectroscopy (EIS) measurements were carried out. Nyquist plots for compound III are shown in Fig. 2 and Bode plots are also shown in Fig. 3.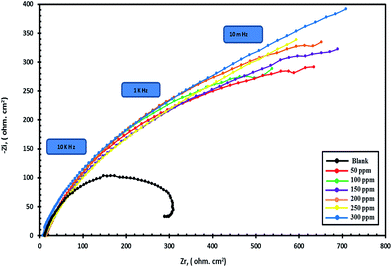 | ||
| Fig. 2 Nyquist plots for carbon steel in sea water in the absence and presence of different concentrations of compound III. | ||
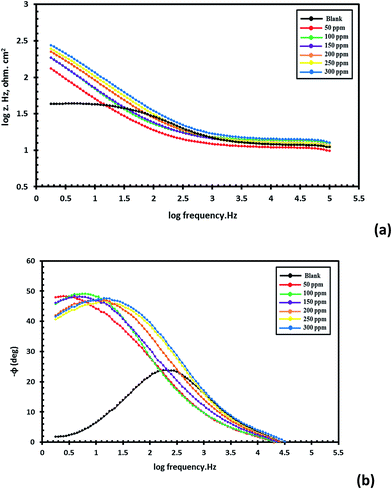 | ||
Fig. 3 Bode plots for carbon steel in sea water in the absence and presence of different concentrations of compound III: (a) log frequency vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z and (b) log frequency vs. phase angle. Z and (b) log frequency vs. phase angle. | ||
The previous plots show that the impedance response of carbon steel in sea water was significantly changed after the addition of the inhibitor molecules. Various parameters such as the charge transfer resistance (Rt), double layer capacitance (Cdl) and percentage inhibition efficiency (IE%) were calculated and listed in Table 5.
| Inhibitor | Conc., ppm | Coefficient | Rs (Ω cm2) | Cs (μF cm2) | Rt (Ω cm2) | Cdl (μF cm2) | IE% |
|---|---|---|---|---|---|---|---|
| Blank | 0 | 0.99 | — | — | 358.13 (±3.5) | 1302 | — |
| (I) | 50 | 0.99 | 230 | 48.1 | 642.00 (±8.0) | 1217 | 44.21 (±0.63) |
| 100 | 0.99 | 270 | 43.4 | 746.33 (±10.0) | 1104 | 52.01 (±1.06) | |
| 150 | 0.99 | 320 | 37.2 | 798.00 (±8.0) | 989.3 | 55.12 (±0.78) | |
| 200 | 0.99 | 350 | 32.5 | 870.33 (±5.0) | 930.1 | 58.85 (±0.58) | |
| 250 | 0.99 | 380 | 29.3 | 1001.00 (±10.0) | 902.5 | 64.22 (±0.61) | |
| 300 | 0.99 | 390 | 28.7 | 1401.00 (±17.7) | 887.4 | 74.43 (±0.51) | |
| (II) | 50 | 0.99 | 250 | 46.2 | 703.00 (±11.8) | 1159 | 49.05 (±0.86) |
| 100 | 0.99 | 290 | 42.4 | 761.45 (±11.7) | 1090 | 52.96 (±0.72) | |
| 150 | 0.99 | 350 | 36.7 | 838.33 (±8.4) | 970.6 | 57.28 (±0.03) | |
| 200 | 0.99 | 370 | 31.1 | 996.00 (±3.0) | 911.8 | 64.04 (±0.42) | |
| 250 | 0.99 | 410 | 28.8 | 1135.33 (±7.1) | 873.1 | 68.46 (±0.12) | |
| 300 | 0.99 | 420 | 27.6 | 1592.00 (±20.4) | 855.7 | 77.50 (±0.20) | |
| (III) | 50 | 0.99 | 280 | 43.2 | 1015.33 (±10.2) | 1004 | 64.73 (±0.37) |
| 100 | 0.99 | 330 | 40.5 | 1203.00 (±12.0) | 978.1 | 70.23 (±0.52) | |
| 150 | 0.99 | 390 | 35.2 | 1356.15 (±12.2) | 914.4 | 73.59 (±0.50) | |
| 200 | 0.99 | 420 | 29.1 | 1799.33 (±6.7) | 877.5 | 80.10 (±0.27) | |
| 250 | 0.99 | 430 | 27.9 | 2364.33 (±8.3) | 822.3 | 84.85 (±0.15) | |
| 300 | 0.99 | 440 | 26.4 | 3987.33 (±9.0) | 786.9 | 91.02 (±0.08) |
The percentage inhibition efficiency IE% was calculated from the values of Rt using the following equation:63
| IE% = [1 − (Rt/Rt(inh))] × 100 | (2) |
3.3. Evaluation of the selected amine derivatives as scale inhibitors
The present work was extended to establish the effectiveness of the selected amine derivatives as scale inhibitors of calcium sulfate deposition in sea water. The laboratory procedures were carried out as described in the Experimental part. The percentage inhibition efficiency was calculated as follow:| Scale inhibition efficiency% = [Cai − Cab/Cac − Cab] × 100 | (3) |
The obtained results are listed in Table 6 and graphically shown in Fig. 5. It is clear that the percentage inhibition efficiency increases with increasing inhibitor concentration, reaching 86.7% at 125 ppm.
| Conc., ppm | Percentage inhibition efficiency (IE%) of inhibitor I | Percentage inhibition efficiency (IE%) of inhibitor II | Percentage inhibition efficiency (IE%) of inhibitor III |
|---|---|---|---|
| 0 | — | — | — |
| 25 | 30.71 (±0.55) | 38.43 (±0.31) | 52.03 (±0.51) |
| 50 | 45.32 (±0.79) | 51.50 (±0.52) | 63.40 (±1.10) |
| 75 | 56.30 (±0.78) | 62.60 (±1.08) | 74.80 (±1.30) |
| 100 | 63.91 (±1.12) | 69.33 (±0.67) | 79.30 (±0.46) |
| 125 | 65.10 (±1.13) | 75.20 (±0.46) | 86.70 (±0.96) |
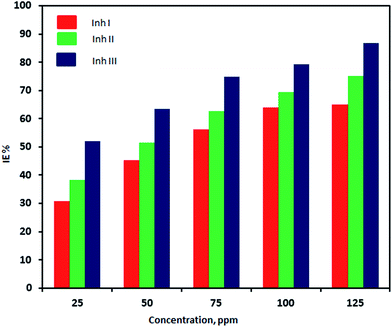 | ||
| Fig. 5 Variation in the efficiency of compounds I, II and III as calcium sulfate scale inhibitors with concentration as determined from ASTM G3-89-re-approved 1994. | ||
3.4. Scanning electron microscopy (SEM)
Fig. 6a shows an SEM image of the polished carbon steel surface. The micrograph shows a characteristic inclusion, which was probably an oxide inclusion.65 Fig. 6b shows an SEM image of the surface of the carbon steel specimen after immersion in sea water for 90 days in the absence of inhibitor, while Fig. 6c shows an SEM image of the surface of another carbon steel specimen after immersion in sea water for the same time interval in the presence of 300 ppm of compound III. The resulting scanning electron micrographs reveal that the surface was strongly damaged in the absence of the inhibitor, but in the presence of 300 ppm of compound III, there was less damage to the surface. This confirms the observed high inhibition efficiency of compound III at this concentration.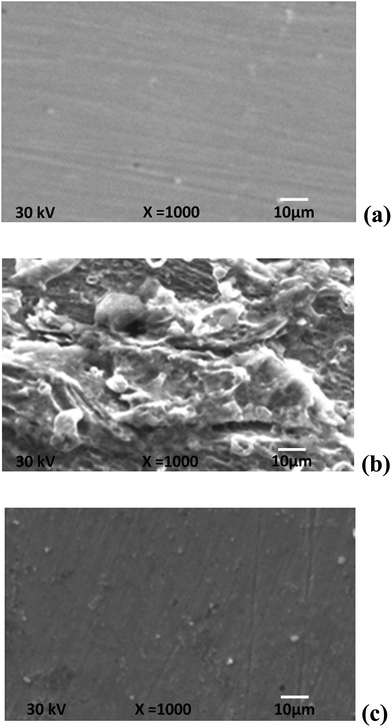 | ||
| Fig. 6 SEM images of the carbon steel surface: (a) polished sample, (b) after immersion in the sea water and (c) after immersion in the sea water in the presence of 300 ppm of compound III. | ||
3.5. Energy dispersive analysis of X-rays (EDX)
The EDX spectrum in Fig. 7a shows the characteristic peaks of some of the elements constituting the polished carbon steel surface. The spectra of the polished carbon steel surface after immersion in sea water in the absence and presence of compound III for 90 days are shown in Fig. 7b and c, respectively. The spectrum in Fig. 7c shows that the Fe peak is considerably decreased relative to the samples shown in Fig. 7a and b. This decrease in the Fe band indicates that a strongly adherent protective film of compound III formed on the polished carbon steel surface, which leads to a high degree of inhibition efficiency.66 EDX and SEM examinations of the carbon steel surface support the results obtained from chemical and electrochemical methods that indicate the compounds are good inhibitors for carbon steel in sea water.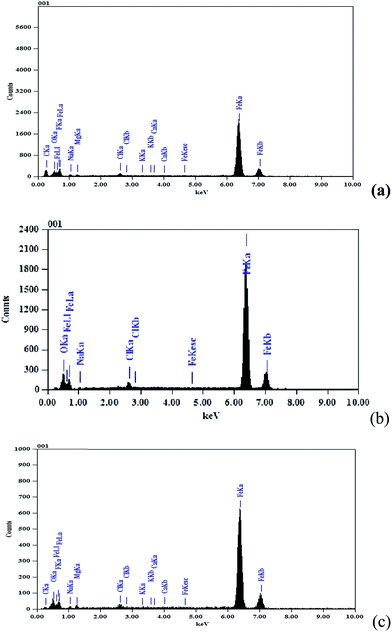 | ||
| Fig. 7 EDX spectra of the carbon steel surface: (a) polished sample, (b) after immersion in sea water and (c) after immersion in sea water in the presence of 300 ppm of compound III. | ||
The results from both the SEM and EDX techniques confirm the formation of a good protective layer on the surface of the carbon steel in the presence of 300 ppm of inhibitor III.
3.6. Inhibition mechanism of the selected amine derivatives
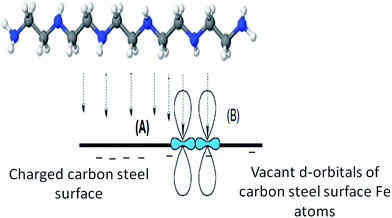 | ||
| Fig. 8 The interaction between pentaethylenehexamine and the carbon steel surface through chemical adsorption processes. | ||
The corrosion inhibition efficiency for inhibitors I–III was tested using potentiodynamic polarization and the EIS technique and follows the sequence:
| III > II > I |
From the obtained results, one can conclude that, as the number of nitrogen atoms in the amine derivatives increases, their inhibition efficiency as a corrosion inhibitor is increased.
4. Conclusions
The results showed that all compounds used in this work act as effective corrosion and scale inhibitors for carbon steel in sea water. The percentage inhibition efficiency (IE%) of the inhibitors increases with the increasing number of ethylene amine units in the inhibitor molecule. EIS data indicates that the value of the charge transfer resistance (Rt) increased with increasing inhibitor concentration, while the value of electrochemical capacitance (Cdl) decreased. The potentiodynamic polarization curves indicated that the inhibitor molecules inhibit both anodic metal dissolution and also cathodic oxygen reduction, so the inhibitors used in this work are classified as mixed type. The inhibition mechanism is attributed to the strong adsorption ability of the selected amine derivatives onto the carbon steel surface, forming a good protective layer, which isolates the surface from its aggressive environment, as confirmed by SEM and EDX techniques.References
- L. L. Sheir and R. A. Jarman, Corrosion, Butterworth-Heinmann Ltd., Great Britain, 3rd edn, 1994 Search PubMed.
- S. N. Banerjee, An introduction to Science of Corrosion and its Inhibition, Oxonian Press PVT Ltd., India, 1985 Search PubMed.
- I. L. Rozenfeld, Corrosion Inhibition, McGraw-Hill Inc., USA, 1981 Search PubMed.
- D. A. Jones, Principles and Prevention of Corrosion, Macmillan Publishing Company, USA, 1991 Search PubMed.
- S. H. You, D. H. Tseng, G. L. Guo and J. J. Yang, The Potential for the Recovery and Reuse of Cooling Water in Taiwan, Resour., Conserv. Recycl., 1999, 26(1), 53–70 CrossRef.
- D.-J. Choi, S.-J. You and J.-G. Kim, Development of an environmentally safe corrosion, scale, and microorganism inhibitor for open recirculating cooling systems, Mater. Sci. Eng., A, 2002, 335, 228–236 CrossRef.
- R. E. Melchers and R. Jeffery, Corros. Rev., 2005, 1, 84 Search PubMed.
- R. E. Melchers and R. Jeffery, Corros. Rev., 2005, 6, 297 Search PubMed.
- G. Saha, N. Kurmaih and N. Hakerman, J. Phys. Chem., 1955, 59, 707 CrossRef.
- P. R. Roberge, Handbook of Corrosion Engineering, McGraw-Hill, 1999 Search PubMed.
- A. Singh, E. E. Ebenso and M. A. Quraishi, Int. J. Electrochem. Sci., 2012, 7, 4766–4779 CAS.
- A. G. Christy, A. Lowe, V. Otieno-Alego, M. Stoll and R. D. Webster, J. Appl. Electrochem., 2004, 34, 25 CrossRef.
- H. Otmacic, J. Telegdi, K. Papp and E. Stupnisek-Lisac, J. Appl. Electrochem., 2004, 34, 545 CrossRef CAS.
- H. Ma, S. Chen, L. Niu, S. Zhao, S. Li and D. Li, J. Appl. Electrochem., 2002, 32, 65 CrossRef CAS.
- F. Zucchi, G. Trabanelli and M. Fonsati, Corros. Sci., 1996, 38, 2019 CrossRef CAS.
- F. Zucchi, G. Trabanelli and N. Alagia, ACH - Models Chem., 1995, 132, 579 CAS.
- C. Wang, S. Chen and S. Zhao, J. Electrochem. Soc., 2004, 151, B11 CrossRef CAS PubMed.
- M. Kendig and S. Jeanjaquet, J. Electrochem. Soc., 2002, 149, B47 CrossRef CAS PubMed.
- H. Y. Ma, C. Yang, B. S. Yin, G. Y. Li, S. H. Chen and J. L. Luo, Appl. Surf. Sci., 2003, 218, 143 CrossRef CAS.
- G. K. Gomma and M. H. Wahdan, Mater. Chem. Phys., 1994, 39, 142 CrossRef CAS.
- K. F. Khaled and N. Hackerman, Electrochim. Acta, 2004, 49, 485 CrossRef CAS PubMed.
- E. Khamis, F. Bellucci, R. Latanision and M. El Ashry, Corrosion, 1991, 47, 677 CrossRef CAS.
- E. Khamis, E. S. H. El Ashry and A. K. Ibrahim, Br. Corros. J., 2000, 35, 150 CrossRef CAS PubMed.
- E. S. H. El Ashry, A. El Nemr, S. A. Esawy and S. Ragab, Electrochim. Acta, 2006, 51, 3957 CrossRef CAS PubMed.
- E. S. H. El Ashry, A. El Nemr, S. A. Esawy, S. Ragab and S. Chem, Indian J. Phys., 2006, 1, 41 CAS , http://pcaij.tsijournals.com.
- D. P. Schweinsberg and V. Ashworth, Corros. Sci., 1988, 28, 539 CrossRef CAS.
- S. N. Raicheva, B. V. Aleksiev and E. I. Sokolova, Corros. Sci., 1993, 34, 343 CrossRef CAS.
- M. A. Quraishi, M. A. Khan, D. Jamal, M. Ajmal, S. Muralidharan and S. Iyer, J. Appl. Electrochem., 1996, 26, 1253 CrossRef CAS.
- M. A. Quraishi, M. Khan, D. Jamal, M. S. Muralidharan and S. V. K. KIyer, Br. Corros. J., 1997, 32, 72 CrossRef CAS PubMed.
- B. Mernari, H. Attari, M. Traisnel, F. Bentiss and M. Lagrenée, Corros. Sci., 1998, 40, 391 CrossRef CAS.
- V. Hluchan, B. L. Wheeler and N. Hackerman, Mater. Corros., 1988, 39, 512 CrossRef CAS PubMed.
- X. L. Cheng, H. Y. Ma, S. H. Chen, R. Yu, X. Chen and Z. M. Yao, Corros. Sci., 1999, 41, 321 CrossRef CAS.
- M. Bouayed, H. Rabaa, A. Schiri, J. Saillard, A. Ben Bachir and A. Le Beuze, Corros. Sci., 1999, 41, 501 CrossRef CAS.
- M. El Azhar, B. Mernari, M. Traisnel, L. Gengembre, F. Bentiss and M. Lagrenée, Corros. Sci., 2001, 43, 2229 CrossRef CAS.
- F. Bentiss, M. Traisnel and M. Lagrenée, J. Appl. Electrochem., 2001, 31, 41 CrossRef CAS.
- L. Wang and G. Yin, Corros. Sci., 2001, 43, 1197 CrossRef CAS.
- M. A. Quraishi, M. Khan, M. Ajmal and S. Muralidharan, Electrochim. Acta, 1995, 13, 63 CAS.
- M. A. Quraishi, M. A. W. Khan, M. Ajmal, S. Muralidharan and S. V. K. Iyer, Corrosion, 1997, 53, 475 CrossRef CAS.
- M. A. Quraishi, M. A. W. Khan and M. Ajmal, Meth. Mater., 1996, 43, 5 CAS.
- A. G. Gad Alla and H. M. Tamous, J. Appl. Electrochem., 1990, 20, 488 CrossRef.
- R. Agrawal and T. K. G. Namboodhiri, Corros. Sci., 1990, 30, 37 CrossRef CAS.
- M. Elayyachy, B. Hammouti and A. El Idrissi, Appl. Surf. Sci., 2005, 249, 176 CrossRef CAS PubMed.
- M. Bouklah, B. Hammouti, M. Lagrenée and F. Bentiss, Corros. Sci., 2006, 48, 2831 CrossRef CAS PubMed.
- M. Ajmal, A. S. Mideen and M. A. Quraishi, Corros. Sci., 1994, 36, 79 CrossRef CAS.
- J. Fang and J. Li, J. Mol. Struct.: THEOCHEM, 2002, 593, 179 CrossRef CAS.
- M. A. Quraishi and H. K. Sharma, Mater. Chem. Phys., 2002, 78, 18 CrossRef CAS.
- F. B. Growcock, N. R. Lopp and R. Jasinski, J. Electrochem. Soc., 1988, 135, 823 CrossRef CAS PubMed.
- R. D. Braun, E. L. Lopez and D. P. Vollmer, Corros. Sci., 1993, 34, 1251 CrossRef CAS.
- T. Szauer and A. Brandt, Electrochim. Acta, 1981, 26, 1209–1219 CrossRef CAS.
- E. S. Lisac, A. Brnada and A. D. Mance, Corros. Sci., 2000, 42, 243–257 CrossRef.
- J. de Damborenea, J. M. Bastidas and A. J. Vhzquez, Electrochim. Acta, 1997, 42, 45459 CrossRef.
- D. Martínez, R. Gonzalez, K. Montemayor, A. J. Hernandez, G. Fajardo and M. A. L. H. Rodriguez, Wear, 2009, 267, 255–258 CrossRef PubMed.
- D. Q. Zhang, L. Gao and G. D. Zhou, Surf. Coat. Technol., 2010, 204, 1646–1650 CrossRef CAS PubMed.
- M. Machado, Cooling tower Technologies and Management – Water Minimization, Australian industry group, 2010 Search PubMed.
- J. Kubis, Power station zero discharge, cooling tower, NACE international, No. 08396, 2010 Search PubMed.
- K. Spiegler and A. Laird, Principles of Desalination, Academic Press, New York, NY, 1980, pp. 667–730 Search PubMed.
- M. Elliot, Scale control by threshold treatment, Desalination, 1970, 8, 221–236 CrossRef CAS.
- K. Cooper, L. Hanlon, G. Smart and R. Talbot, The threshold inhibition phenomenon, Desalination, 1979, 31, 257–266 CrossRef CAS.
- K. M. Ismail, Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions, Electrochim. Acta, 2007, 52, 7811–7819 CrossRef CAS PubMed.
- NACE standard TM 0374–2001.
- J. Zhang, Z. P. Li, W. M. Zhao, W. Y. Guo and Y. Wang, Pet. Process. Sect., 2008, 21(24), 995–998 Search PubMed.
- Q. B. Zhang and Y. X. Hua, Electrochim. Acta, 2009, 54, 1881 CrossRef CAS PubMed.
- A. P. Yadav, A. Nishikata and T. Tsuru, Corros. Sci., 2004, 46, 169 CrossRef CAS.
- K. F. Khaled, Appl. Surf. Sci., 2006, 252, 4120 CrossRef CAS PubMed.
- ASTM E 45–87, ASTM, Philadelphia, PA, 1980, vol. 11, p. 125 Search PubMed.
- M. A. Amin, J. Appl. Electrochem., 2006, 36, 215 CrossRef CAS PubMed.
- M. Behpour, S. M. Ghoreishi, M. Salavati-Niasari and B. Ebrahimi, Mater. Chem. Phys., 2008, 107, 153–157 CrossRef CAS PubMed.
- A. Yurt, A. Balaban, S. UstunKandemir, G. Bereket and B. Erk, Mater. Chem. Phys., 2004, 85, 420–426 CrossRef CAS PubMed.
- L. Vracar and D. M. Drazic, Corros. Sci., 2002, 44, 1669–1680 CrossRef CAS.
- I. Ahamad, R. Prasad and M. A. Quraishi, Corros. Sci., 2010, 52, 1472–1481 CrossRef CAS PubMed.
- A. E. Martel and M. Calvin, Chemistry of Metal Chelate Compounds, Prentic-hall Inc., New York, USA, 1952 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11082c |
| This journal is © The Royal Society of Chemistry 2015 |