Bio-based nickel alginate and copper alginate films with excellent flame retardancy: preparation, flammability and thermal degradation behavior
Abstract
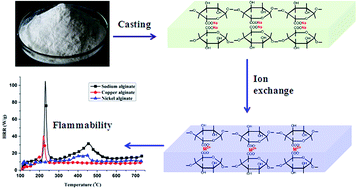
A bio-based nickel alginate film and copper alginate film were prepared via a facile ion exchange and casting approach. Their flame retardancy, thermal degradation and pyrolysis behavior, and thermal degradation mechanism were investigated systematically by the limiting oxygen index (LOI), vertical burning (UL-94), microscale combustion calorimetry (MCC), thermogravimetric analysis (TGA), thermogravimetric analyzer coupled with Fourier transform infrared analysis (TG-FTIR) and pyrolysis-gas chromatography-mass spectrometry (Py-GC-MS). It was shown that the nickel alginate film had a much higher LOI value (50.0%) than those of the sodium alginate film (24.5%) and copper alginate film (23.0%). Moreover, the nickel alginate film passed the UL-94 V-0 rating, while the sodium alginate film and copper alginate film showed no classification. Importantly, the peak of heat release rate (PHRR) of the nickel alginate film in the MCC test was much lower than those of the copper alginate film and sodium alginate film. This indicated that the introduction of nickel ions decreased the release of combustible gases. TGA results showed that the addition of copper ions and nickel ions accelerated the thermal degradation of alginates and changed the thermal degradation mechanism of the alginates. TG-FTIR and Py-GC-MS results indicated that the pyrolysis of copper alginate and nickel alginate produced much less flammable products than that of sodium alginate in the whole thermal degradation process. Finally, a possible degradation mechanism for copper alginate and nickel alginate was proposed. The results of our study provide useful information for understanding the flame retardancy mechanism of alginate as well as for designing bio-based materials with excellent fire retardancy.

 Please wait while we load your content...
Please wait while we load your content...