Chromogenic ‘naked eye’ and fluorogenic ‘turn on’ sensor for mercury metal ion using thiophene-based Schiff base†
Divya Singhal,
Neha Gupta and
Ashok Kumar Singh*
Department of Chemistry, Indian Institute of Technology-Roorkee, Roorkee-247667, India. E-mail: akscyfcy@iitr.ernet.in
First published on 23rd July 2015
Abstract
2-((3-Methylthiophen-2-yl)methyleneamino)benzenethiol (Probe 1) has been synthesized and successfully applied for the selective recognition of mercury metal ions and utilized as a fluorescence turn-on sensor for Hg2+ ion via chelation enhanced fluorescence (CHEF). The mechanism of the interaction of Probe 1 with metal ions has been deduced by the absorption, emission, 1H-NMR, and MALDI-TOF Mass analysis that intimate the favourable coordination of Hg2+ metal ion by the mercapto unit. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the sensor complex 1 + Hg2+ was calculated from the Job’s plot based on UV-Vis absorption spectra. The binding constant (log
1 stoichiometry of the sensor complex 1 + Hg2+ was calculated from the Job’s plot based on UV-Vis absorption spectra. The binding constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2) of the 1 + Hg2+ complex was found to be 13.36 by Hill plot. The limit of detection (LOD) was also calculated from the fluorescence emission titration and found to be 20 μM. Moreover the density functional theory (DFT) studies were investigated for the 1 + Hg2+ binding mechanism. Cyclic voltammograms also confirmed the recognition of Hg2+ metal ion to the Probe 1.
β2) of the 1 + Hg2+ complex was found to be 13.36 by Hill plot. The limit of detection (LOD) was also calculated from the fluorescence emission titration and found to be 20 μM. Moreover the density functional theory (DFT) studies were investigated for the 1 + Hg2+ binding mechanism. Cyclic voltammograms also confirmed the recognition of Hg2+ metal ion to the Probe 1.
1. Introduction
Many human disorders are caused by the exposure of the toxic metal ion: like mercury, lead, cadmium, silver. Many efforts have been devoted to the development of fluorogenic and colorimetric sensors for the highly toxic mercury ion. The mercury metal ion is highly toxic, non-biodegradable,1 hazardous in nature,2–4 and toxic for humans, including brain, kidney and lung damage. The results of mercury poisoning in several diseases, including acrodynia, and Minamata disease. The toxicity of Hg2+ in humans is caused by the easy coordination with biological ligands such as proteins, DNA and enzymes due to its affinity towards thiol groups. It's present significant hazards to public health because of its presence in drinking water. Therefore, the development of rapid, cost-effective and enzyme-free colorimetric sensors for the easy and fast detection of toxic metal ions by the naked eye, without resorting to any expensive instruments, is still an active ongoing research area.The sources of Hg2+ contamination in water include gold mining, rubber processing, fertilizer industries, oil refining, and wood pulping. All the different forms of mercury ion, such as zero oxidation, mercuric ion in Hg2+, and mercurous in Hg22+, have toxic effects on the environment. Due to the bioaccumulation and magnification of Hg2+ in the aquatic food chain, Hg2+ has serious environmental toxicity. So construction of a chemosensor for Hg2+ ion detection is in demand. Due to the low level of metals in the samples and complexity of the matrices, the analysis of metal ions in the environment, clinical, industrial and biological samples is still a challenging task. Many sensors have been reported based on a fluorescence ‘turn-off’ response due to the quenching nature of heavy metal Hg2+ ions having large spin–orbit coupling constants.5–9 And recently, several ‘turn-on’ fluorescence sensors for Hg2+ ion have been reported.10–15 However, chemosensors to detect Hg2+ ion via enhanced fluorescence are very rare. We herein report a new sensor motif to detect Hg2+ through a fluorescence “turn-on” response in a partially aqueous medium.
The World Health Organization (WHO) and Environmental Protection Agency (EPA) have defined the limited concentration of these metal ions in drinking water. There are many important techniques which facilitate the quantification of these metal ions like: Atomic Absorption Spectroscopy (AAS) and ICP-MS.16–19 Due to their high cost and high maintenance, colorimetric sensors have been developed. For the recognition of soft, heavy metal Hg2+ ions, nitrogen and sulphur binding sites might be a choice as they are present in the thiophene-based Schiff base.
2. Experimental section
2.1. Reagents and instrumentation
Chloride and nitrate salts of metal ions were all of analytical reagent grade and purchased from Merck. These reagents were used without further purification. 3-Methylthiophene-2-carbaldehyde, 2-aminothiophenol, 3-aminophenol were purchased from Sigma-Aldrich. The UV-Vis analysis of all the solutions was recorded on a Shimadzu, UV-3600 double beam spectrophotometer using a 10 mm path length silica cell. IR spectra were recorded with a Perkin-Elmer FT-IR 1000 spectrophotometer as films between KBr. CHNS analysis was recorded on an Elementar model Vario EL-III. NMR spectra were recorded on a Bruker AVANCE 500 MHz spectrometer. MALDI-TOF mass spectra were recorded on a Bruker Ultra-fleXtreme-TN-MALDI-TOF spectrometer using HABA (2-(4-hydroxyphenylazo)benzoic acid) as a matrix. Fluorescence emission spectra were recorded using RF-5301PC with a standard quartz cell of 3 cm path length. Cyclic voltammetric studies were carried out at room temperature on a CHI760E electroanalyser. The potential range was +1.5000 V to −1.5000 V at a scan rate of 0.1 V s−1 using a glassy carbon electrode as the working electrode, a Ag/AgCl electrode as the reference electrode and Pt wire as the auxiliary electrode, and 0.1 M tetrabutylammonium perchlorate (TBAP) was used as the supporting electrolyte. All solutions were purged with nitrogen before the experiment. DFT computation studies were organized in the Gaussian 09 W programme in gas phase using a B3LYP function with 6-31G (d, p) for the metal-free ligand and LANL2DZ for the metal–ligand complex.2.2. Synthesis of Probe 1 and 2
Yield: 77%. Anal. calc. for C12H11NS2: C, 61.76; H, 4.75; N, 6.00, S, 27.48 found: C, 62.01; H, 4.50; N, 6.71; S, 26.99. IR data (KBr, νmax cm−1): Ar–H: 3117, S–H: 2359, C–N: 1568, C–C: 1414, C–O: 1247. UV-visible (MeOH, λmax nm−1): 287, 355. 1H NMR (DMSO, 500 MHz, δ/ppm): 8.78 (s, 1H), 7.36 (d, 1H), 7.21 (d, 1H), 7.10 (t, 1H), 6.92 (dd, 1H), 6.87 (d, 1H), 6.83 (t, 1H), 4.66 (s, 1H), 2.44 (s, 3H), 13C NMR (DMSO, 125 MHz, δ/ppm) 151, 148, 142, 136, 135, 131, 130, 128, 120, 115, 114, 83.
Yield: 62%. Anal. calc. for C12H11NOS: C, 66.33; H, 5.10; N, 6.45; O, 7.36; S, 14.76 found: C, 66.10; H, 4.83; N, 6.79; O, 8.72; S, 13.56 IR data (KBr, νmax cm−1): O–H: 3433, C–N: 1607, C–C: 1422, UV-visible (MeOH, λmax nm−1): 383. 1H NMR (DMSO, 500 MHz, δ/ppm): 8.65 (s, 1H), 7.38 (d, 1H), 7.24 (d, 1H), 6.95 (m, 4H), 3.19 (broad s, 1H), 2.48 (s, 3H), 13C NMR (DMSO, 125 MHz, δ/ppm) 158, 150, 144, 141, 138, 136, 134, 131, 129, 122, 114, 55.
The structures of both the Schiff bases are shown in Scheme 1. The characterization data of Probe 1 and 2 are shown in the ESI (Fig. S1 to S6†).
3. Results and discussion
Mercury ions have a binding affinity with the sulphur atom because of the soft ligand. For this purpose, we proposed the synthesis of a ligand with a mercapto unit and explored the selective recognition of mercury ions. Mercury binds with the ligand moiety in a linear pattern.3.1. Naked eye detection of metal ion
To investigate the metal ion recognition with the synthesized Schiff bases (Probe 1 and 2), colorimetric studies were carried out with various metal ions. The solution of all the metal ions and Probes were prepared (50 mM) in methanol/H2O (8/2: v/v solution). 3 equivalents of metal solution were added to the Probe 1 solution, and the sudden color change of Probe 1 from light yellow to yellowish orange was observed with the Hg2+ ion (Fig. 1). Furthermore, the selectivity of Probe 1 towards mercury ions was confirmed by UV-Vis and fluorescence studies. Probe 2 did not show any change in color with different metal ions. | ||
| Fig. 1 Images of the colorimetric changes of Probe 1 with Hg2+ ions in a methanol/H2O (8/2; v/v) solution. | ||
3.2. UV-Vis studies of Probe 1 and 2 with metal ions
To investigate the selective recognition of Hg2+ with Probe 1, UV-Vis studies were carried out in methanol/H2O (8/2: v/v solution). Probe 1 showed absorption peaks at 287 nm due to a π–π* transition and at 355 nm due to an n–π* transition. Upon the addition of mercury solution to the Probe 1 solution (50 mm), a new absorption band occurred at 430 nm in the UV-Vis spectra due to the metal recognition (Fig. 2a), and the other metal ions did not show any significant changes in the UV-Vis spectra with Probe 1. Probe 2 did not show any absorption changes upon addition of the metal ions (Fig. 2b).To know the binding stoichiometry of the 1 + Hg2+ complex, equimolar solutions of Probe 1 and Hg2+ ions were prepared (50 μM in methanol/H2O (8/2: v/v solution)) and the absorption spectra were taken by a continuous variation of 1 and Hg2+ solution.
Fig. 3a shows the changes in the absorption spectra and Job’s plot,20 which show the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. UV-Vis spectra were shown to change with the addition of Hg2+ ion in Probe 1 (100 μM), a concomitant increase in the absorbance at 430 nm and decreased in the absorbance at 355 nm and 287 nm were observed. The formation of the isosbestic point confirmed (Fig. 3b) the change from uncomplexed species (Probe 1) to complex species (1 + Hg2+). The red shift in the UV-Vis spectra (90 nm) was responsible for the sudden color change of the solution of Probe 1, i.e., light yellow to yellowish orange upon the addition of Hg2+ ions. The new absorption band occurred due to the intramolecular charge transfer between Probe 1 and mercury metal ions.
1 stoichiometry. UV-Vis spectra were shown to change with the addition of Hg2+ ion in Probe 1 (100 μM), a concomitant increase in the absorbance at 430 nm and decreased in the absorbance at 355 nm and 287 nm were observed. The formation of the isosbestic point confirmed (Fig. 3b) the change from uncomplexed species (Probe 1) to complex species (1 + Hg2+). The red shift in the UV-Vis spectra (90 nm) was responsible for the sudden color change of the solution of Probe 1, i.e., light yellow to yellowish orange upon the addition of Hg2+ ions. The new absorption band occurred due to the intramolecular charge transfer between Probe 1 and mercury metal ions.
3.3. Fluorescence emission spectra with metal ions
All the experiments were carried out in methanol/H2O. Initially, Probe 1 (20 μM) was in methanol/H2O; a 8/2 v/v solution was tested with 60 μM (3 equiv.) of metal ions (Na+, K+, Fe2+, Cr3+, Ni2+, Co2+, Zn2+, Cd2+, Pb2+, Mg2+, Cu2+, Mn2+, Hg2+, and Ag+).Upon treating Probe 1 with 3 equiv. of metal ions, a turn-on emission occurred at 503 nm with Hg2+ ions (λex = 365 nm) (Fig. 4). The ‘turn on’ fluorescence selectivity of Hg2+ ions was caused by chelation enhanced fluorescence (CHEF). The covalent bond formation between the –SH group and Hg2+ ions exhibited the enhanced fluorescence response.
 | ||
| Fig. 4 Fluorescence spectra of Probe 1 (5 μM) towards different metal ions in methanol/water (8/2 v/v solution) (λex = 365 nm). | ||
From the UV-Vis studies and fluorescence studies, it was found that the Probe 1 is highly selective for Hg2+ ions (Table 1). To confirm this selectivity, a single and dual metal study has done with Probe 1. In the course of the dual-metal studies, two equal amounts of both metal ion Hg2+ and other metal ions (60 μM + 60 μM) were used. The interference effect of the secondary metal ion for the selectivity of Hg2+ ions was also carried out and is shown in Fig. 5. The blue bar indicates the single metal ions (Na+, K+, Fe2+, Cr3+, Ni2+, Fe2+, Co2+, Zn2+, Cd2+, Pb2+, Mg2+, Cu2+, Mn2+, Hg2+, and Ag+) with Probe 1 and the red bar indicates the 1 + Hg2+ with interfering ions. Fig. 5 shows the enhanced fluorescence intensity with Hg2+ ions. No other metal ions showed an enhancement in fluorescence intensity. No metal ions interfered with the selectivity of Hg2+ (red bar). So with the help of the interference study, it was confirmed that Probe 1 was highly selective and sensitive for Hg2+ metal ions.
| Sample | UV (nm) study | Fluorescence study | LOD by fluorescence titration |
|---|---|---|---|
| Probe 1 | 287, 355 nm | — | — |
| 1 + Hg2+ | 287, 355, 430 nm | 503 nm enhanced fluorescence | 20 μM |
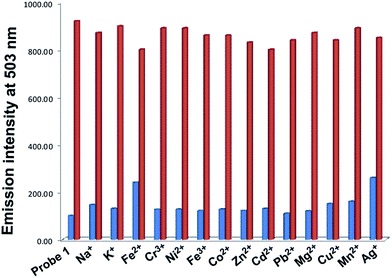 | ||
| Fig. 5 Interference study with different metal ions at 503 nm in methanol/H2O solution, Probe 1 + metal ions (blue bar) and 1 + metal ions + Hg2+ (red bar). | ||
3.4. Fluorescence titration21,22 of Hg2+ ions with Probe 1
For the fluorescence titration, 10 μL aliquots of Hg2+ ions of fixed concentrations were added to a 3 mL solution of Probe 1 (Fig. 6). The binding constant was calculated by fluorescence titration using the Hill method.23 Hill plot was constructed by plotting graph between log[(I − I0)/(Imax − I)] against log(Hg2+), where I0, I and Imax are the emission intensity values of Probe 1, 1 + Hg2+ complex and after the complete binding of 1 + Hg2+ complex respectively. | ||
| Fig. 6 Fluorescence emission titration of Probe 1 with various concentrations of Hg2+ metal ions. Inset shows Hill plot. | ||
The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 value for the Hg2+ ions with Probe 1 was found to be 13.36 with a 20 μM limit of detection (LOD)24 (Fig. 7), which is lowered than previously reported in the literature.
β2 value for the Hg2+ ions with Probe 1 was found to be 13.36 with a 20 μM limit of detection (LOD)24 (Fig. 7), which is lowered than previously reported in the literature.
3.5. pH studies
The optical behaviour of Probe 1 with Hg2+ ion was evaluated at varying pH by absorption and emission spectroscopy. All the above analysis was carried out at a neutral pH. In the absorption analysis, the spectra remained the same at pH 6–10, but in a more alkaline medium (pH 11–14), disappearance of the absorption band at 421 nm was observed, which was due to the intramolecular charge transfer between Probe 1 and the Hg2+ ions. At low pH 1–5 a total blue shift occurred (Fig. 8). In the emission spectra, Probe 1 showed enhancement in the intensity with Hg2+ ions, accordingly at pH 7–11. Probe 1 with Hg2+ at pH 1–6 illustrated a marginal enhancement in the emission intensity at 503 nm and a decrease in the emission intensity as we increased the pH to 12 to 14 (Fig. 9). The whole study suggested that the chemosensor was properly used for the recognition of Hg2+ ions in pH 7–10.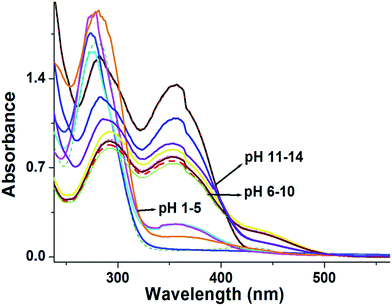 | ||
| Fig. 8 Variations in the absorption spectra of Probe 1 with Hg2+ ions at different pH in MeOH/H2O solution. | ||
 | ||
| Fig. 9 Variation in the emission spectra of Probe 1 with Hg2+ ions at different pH in MeOH/H2O solution. | ||
3.6. Stoichiometry of binding25
Stoichiometry is an important tool for sensing mechanisms. To find out the binding site of Probe 1, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of the 1 + Hg2+ complex was calculated using a Job’s plot. By the Job’s plot it was confirmed that the absorption maxima occurred at 0.66 mole fractions, which facilitated the 2
1 stoichiometry of the 1 + Hg2+ complex was calculated using a Job’s plot. By the Job’s plot it was confirmed that the absorption maxima occurred at 0.66 mole fractions, which facilitated the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry complex.
1 stoichiometry complex.
3.7. Nature of binding interactions
To sustain this hypothesis of the interaction of Probe 1 towards Hg2+ ions, 1H-NMR studies were carried out. The 1H-NMR spectra of Probe 1 in CDCl3 showed a sharp singlet at δ 8.78 ppm of –CH protons (Ha) and doublets and triplets were assigned to aromatic protons. The resonance signal appeared at δ 4.66 and 2.44 ppm and were assigned to the –SH proton and –CH3 proton, respectively. After the addition of Hg2+ ions, the downfield shift of the aromatic protons (Δδ = 0.02) occurred due to the interaction of a π–el− cloud of aromatic rings to the Hg2+ metal ions, and a significant downfield shift of the resonance signal of the –SH proton occurred. With an increase in the concentration of Hg2+ ions the –SH proton signal completely disappeared. The 1H NMR studies, as discussed thus, clearly suggested that the complexation occurred after complete deprotonation of the mercapto proton.Moreover, to confirm the stoichiometry of the 1 + Hg2+ complex, MALDI-TOF spectra were recorded (Fig. S7 in the ESI†), which showed a molecular ion peak, m/z at 667.356 (calcd 667.022). 1H NMR spectral data analysis of a complex, 1 + Hg2+ and MALDI-TOF spectra clearly suggested the interaction of Probe 1 with Hg2+ through the S atoms in the mercapto unit in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (Fig. 10).
1 stoichiometry (Fig. 10).
3.8. Computational studies
Theoretical calculations26 were conducted using a density functional theory (DFT) method. Geometry was optimized in gas phase using a Gaussian 09 W computational program with a B3LYP function by the basis set 6-31G (d, p) for the metal-free ligand and LANL2DZ for the metal bound ligand. Computational studies of Probe 1 and 1 + Hg2+ also confirmed the ligand to metal charge transfer. Fig. 11 shows the optimized structure of Probe 1 and 1 + Hg2+ and the HOMO–LUMO band gap, which confirmed the ligand to metal charge transfer. To find out the mechanism of binding interaction of Probe 1 with Hg2+, the optimized structure of 1 + Hg2+ indicated that the S–Hg–S linkage formation had a bond angle of 179.9°. Mercury metal forms a complex with the Schiff base in a linear fashion. The HOMO–LUMO band gap of the 1 + Hg2+ complex was decreased because of the ligand to metal charge transfer occurring between Probe 1 and the Hg2+ ions. The optimized structure and HOMO–LUMO diagram of Probe 2 is shown in Fig. S8.†3.9. Cyclic voltammetry
Further, the electrochemical behavior of Probe 1, Probe 2 and Probe 1 with the Hg2+ ion was tested in methanol solution. The cyclic voltammograms of Probe 1 show two irreversible reduction peaks and three oxidation peaks. The reduction peaks occurred at −1.22 and −0.635 V and the oxidation peaks at 0.202, 0.516 and 1.07 V. After the addition of a methanolic solution of Hg2+ ions, the first reduction potential of Probe 1 was showed a 0.131 V cathodic shift. The first oxidation peak showed a 0.373 V anodic shift. Probe 1 showed a higher oxidation potential after binding with Hg2+ ions due to the charge transfer from the ligand to metal ions (Fig. 12). These results are also supported by theoretical calculations. All the CV data are summarised in Table 2. The cyclic voltammograms of Probe 2 had one irreversible reduction peak and two irreversible oxidation peaks. The reduction peak occurred at −0.60 V and the oxidation peaks occurred at 0.88 and 1.30 V. The cyclic voltammograms of Probe 2 are mentioned in the ESI (Fig. S9†).| Sample | Oxidation peak (V) | Reduction peak (V) | |||
|---|---|---|---|---|---|
| Probe 1 | 1.07 | 0.516 | 0.202 | −0.635 | −1.22 |
| 1 + Hg2+ | 1.13 | 0.852 | 0.575 | −0.766 | −1.19 |
| Probe 2 | 1.30 | 0.88 | — | −0.60 | — |
3.10. Reversibility of the proposed sensor27
The reversible behaviour of the proposed sensor was checked with EDTA disodium salt. Probe 1 showed the ‘turn-on’ fluorescence emission at 503 nm with Hg2+ metal ions in methanol/H2O (8/2: v/v solution). After the addition of 10 mM of EDTA (in water) into the 1 + Hg2+ solution (yellowish orange color), the fluorescence enhanced emission was quenched and the color disappeared. Furthermore, the solution was reused for the chemosensing of Hg2+ metal ions. The fluorescence intensity and reversible colorimetric images are shown in Fig. 13.The proposed chemosensor for Hg2+ metal ions was compared with those previously reported in the literature. From Table 3, the proposed sensor results seem good with respect to the limit of detection (LOD).
| Previous literature | Solvent | Limit of detection (LOD) |
|---|---|---|
| Dalton Trans., 2013, 42, 4456 (ref. 28) | CH3CN | 50 mM |
| Org. Biomol. Chem., 2012, 10, 5410 (ref. 29) | H2O–CH3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v) 90, v/v) |
0.226 mM |
| Tetrahedron Lett., 2010, 51, 3286 (ref. 30) | CH3CN–DDW | 30 mM |
| Spectrochim. Acta, Part A, 2012, 93, 245 (ref. 31) | DMSO | 5.0 mM |
| Org. Lett., 2010, 12, 476 (ref. 32) | C2H5OH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) 1; v/v) |
80 μM |
| This work | CH3OH/H2O (8/2; v/v) | 20 μM |
4. Conclusion
Thiophene-based Probes (1 and 2) have been synthesised and characterised by various spectroscopic techniques such as UV-Vis, FT-IR, and NMR. Furthermore, Probe 1 was used for fluorometric and colorimetric sensing of Hg2+ metal ions without any interference from other metal ions. The binding affinity of Hg2+ ions with Probe 1 was also confirmed by the optical studies, NMR, mass studies, DFT optimization and electrochemical behaviour. The designed chemosensor was accomplished for the detection of 20 μM Hg2+ ions in a partially aqueous medium.Acknowledgements
Ms Divya Singhal is highly grateful to CSIR (Council Scientific of Industrial Research) for providing funding for this work.References
- Z. Yan, M. Yuen, L. Hu, P. Sun and C. Lee, RSC Adv., 2014, 4, 48373 RSC.
- A. Renzoni, F. Zino and E. Franchi, Environ. Res., 1998, 77, 68 CrossRef CAS PubMed.
- A. T. Wright and E. V. Anslyn, Chem. Soc. Rev., 2006, 35, 14 RSC.
- X. Zhang, Y. Xiao and X. Qian, Angew. Chem., Int. Ed., 2008, 47, 8025 CrossRef CAS PubMed.
- H. Lee, H. S. Lee, J. H. Reibenspies and R. D. Hancock, Inorg. Chem., 2012, 51, 10904 CrossRef CAS PubMed.
- G. Aragay, J. Pons and A. Merkoci, Chem. Rev., 2011, 111, 3433 CrossRef CAS PubMed.
- H. Wang and S. Wu, Tetrahedron, 2013, 69, 1965 CrossRef CAS PubMed.
- P. Srivastava, R. Ali, S. S. Razi, M. Shahid, S. Patnaik and A. Misra, Tetrahedron Lett., 2013, 54, 3688 CrossRef CAS PubMed.
- P. Srivastava, R. Ali, S. S. Razi, M. Shahid and A. Misra, Sens. Actuators, B, 2013, 181, 584 CrossRef CAS PubMed.
- Y. Chen, C. Zhu, Z. Yang, J. Li, Y. Jiao, W. He, J. Chen and Z. Guo, Chem. Commun., 2012, 48, 5094 RSC.
- Z. Xie, K. Wang, C. Zhang, Z. Yang, Y. Chen, Z. Guo, G. Y. Lu and W. He, New J. Chem., 2011, 35, 607 RSC.
- P. Das, A. Ghosh, H. Bhatt and A. Das, RSC Adv., 2012, 2, 3714–3721 RSC.
- A. Misra and M. Shahid, J. Phys. Chem. C, 2010, 114, 16726 CAS.
- P. Srivastava, S. S. Razi, R. Ali, R. C. Gupta, S. S. Yadav, G. Narayan and A. Misra, Anal. Chem., 2014, 86, 8693 CrossRef CAS PubMed.
- P. Srivastava, M. Shahid and A. Misra, Org. Biomol. Chem., 2011, 9, 5051 CAS.
- T. Zhang, G. She, X. Qi and L. Mu, Tetrahedron, 2013, 69, 7102 CrossRef CAS PubMed.
- K. Tayade, B. Bondhopadhyay, A. Basu, G. Krishna Chaitanya, S. K. Sahoo, S. Attarde, N. Singh and A. Kuwar, Talanta, 2014, 122, 16 CrossRef CAS PubMed.
- R. M. de Jesus, L. O. B. Silva, J. T. Castro, A. D. de Azevedo Neto, R. M. de Jesus and S. L. C. Ferreira, Talanta, 2013, 106, 293 CrossRef CAS PubMed.
- M. Noël, J. R. Christensen, J. Spence and C. T. Robbins, Sci. Total Environ., 2015, 529, 1 CrossRef PubMed.
- P. Job, Ann. Chim., 1938, 115, 332 Search PubMed.
- D. Mahajan, N. Khairnar, B. Bondhopadhyay, S. K. Sahoo, A. Basu, J. Singh, N. Singh, R. Bendre and A. Kuwar, New J. Chem., 2015, 39, 3071 RSC.
- M. Shellaiah, Y. C. Rajan, P. Balu and A. Murugan, New J. Chem., 2015, 39, 2523 RSC.
- H. Fang, M. Shellaiah, A. Singh, M. V. R. Raju, Y. Wu and H. Lin, Sens. Actuators, B, 2014, 194, 229 CrossRef CAS PubMed.
- J. Li, Y. Wu, F. Song, G. Wei, Y. Cheng and C. Zhu, J. Mater. Chem., 2012, 22, 478 RSC.
- (a) Y. Q. Weng, F. Yue, Y. R. Zhong and B. H. Ye, Inorg. Chem., 2007, 46, 7749 CrossRef CAS PubMed; (b) M. H. Yang, C. R. Lohani, H. Cho and K. H. Lee, Org. Biomol. Chem., 2011, 9, 2350 RSC.
- H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- V. Bhalla, V. Vij, R. Tejpal, G. Singh and M. Kumar, Dalton Trans., 2013, 4456 RSC.
- (a) N. Niamnont, N. Kimpitak, K. Wongravee, P. Rashatasakhon, K. K. Baldridge, J. S. Siegel and M. Sukwattanasinitt, Chem. Commun., 2013, 49, 780 RSC; (b) S. Sirilaksanapong, M. Sukwattanasinitt and P. Rashatasakhon, Chem. Commun., 2012, 48, 293 RSC.
- M. Vedamalai and S. P. Wu, Org. Biomol. Chem., 2012, 10, 541 Search PubMed.
- S. K. Kim, K. M. K. Swamy, S. Y. Chung, H. N. Kim, M. J. Kim, Y. Jeong and J. Yoon, Tetrahedron Lett., 2010, 51, 3286 CrossRef CAS PubMed.
- J. Liu, M. Yua, X. Wang and Z. Zhang, Spectrochim. Acta, Part A, 2012, 93, 245 CrossRef CAS PubMed.
- J. Du, J. Fan, X. Peng, P. Sun, J. Wang, H. Li and S. Sun, Org. Lett., 2010, 12, 476 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra11043b |
| This journal is © The Royal Society of Chemistry 2015 |








