Synthesis and formation mechanism of VO2(A) nanoplates with intrinsic peroxidase-like activity†
Liangmiao Zhang‡
a,
Fang Xia‡bc,
Zhengdong Songd,
Nathan A. S. Webstere,
Hongjie Luoad and
Yanfeng Gao*d
aShanghai Institute of Ceramics (SIC), Chinese Academy of Sciences (CAS), 1295 Dingxi Rd., Shanghai 200050, China
bCSIRO Manufacturing Flagship, Clayton, VIC 3168, Australia
cSchool of Engineering and Information Technology, Murdoch University, Murdoch, WA 6150, Australia
dSchool of Materials Science and Engineering, Shanghai University, 99 Shangda Rd., Shanghai 200444, China. E-mail: yfgao@shu.edu.cn
eCSIRO Mineral Resources Flagship, Private Bag 10, Clayton South, VIC 3169, Australia
First published on 9th July 2015
Abstract
Monocrystalline VO2(A) nanoplates have been synthesized via a one-pot hydrothermal process. In situ powder X-ray diffraction was used to monitor the hydrothermal synthesis and it was found that VO2(A) nucleates and grows directly from solution after the complete hydrolysis of a 2.0 M VO(acac)2 precursor solution, rather than involving a previously reported intermediate phase VO2(B). A hydrating–exfoliating–splitting mechanism was established to explain the formation of the nanoplate architecture. The synthesized VO2(A) nanoplates showed outstanding peroxidase-like activity and hence are a promising candidate for artificial peroxidase.
1. Introduction
Vanadium oxides and their metastable structures have received considerable attention because of their diverse structures and novel physico-chemical properties. Up to now, several crystalline phases of VOx and V–O hydrates with oxidation states from +2 to +5 have been reported, including monoclinic VO,1 V2O3,2 VO2(M),3 rutile VO2(R),4 tetragonal VO2(A),5 monoclinic VO2(B),6 VO2(C),7 VO2(D),8 paramontroseite VO2(P),9 V3O7·H2O,10 VO2·0.5H2O,11 V2O4·0.25H2O,12 haggite V4O6(OH)4,13 and VOOH (montroseite, orthorhombic and hollandite-type phases).14–16 Among them, monoclinic VO2(M) (space group P21/c) and tetragonal VO2(R) (P42/mnm) undergo reversible phase transformation at ∼67 °C, accompanied by dramatic changes in electrical resistance and optical transmittance.17 These properties enable a wide range of applications in smart windows, gas sensors, optoelectronic switching devices, Mott field-effect transistors, for example.18 Another thermodynamically metastable polymorph VO2(B) (C2/m) is the most common phase formed from solution synthesis. It consists of layers of distorted corner and edge-sharing VO6 octahedra which form a tunnel structure. It has also attracted much interest in the last few years owning to its proper work potential as well as its high energy capacity when used as electrode materials in aqueous Li-ion batteries (LIBs).19 As is well known, the traditional intercalation compound VO2(A) shows similar layered structure as VO2(B) except for the distorted VO6 octahedra in VO2(B) and different stack mode between them. However, research on other metastable phases, such as layered VO2(A), has been rarely reported.Théobald first discovered the existence of VO2(A) as an intermediate phase in a study of hydrothermal reaction in the V2O3–V2O5–H2O system.20 Later, the crystal structure and phase transition were thoroughly investigated by Oka.21,22 More recently, various VO2(A) nanostructures have been successfully fabricated. For example, Ji and coworkers selectively prepared VO2(A) by controlling the pressure during the hydrothermal synthesis.23 Li and coworkers reported the ultra-long VO2(A) nanobelts synthesized hydrothermally using V2O5 sol as precursor and polyethylene glycol as both surfactant and reducing agent.24 Xie et al. reported the large-scale fabrication of VO2(A) nanobelts by simple hydrolysis of VO(acac)2 and observed an intermediate phase VO2(B).25 In addition, Dai et al. prepared 1D VO2(A) nanostructures via a one-step hydrothermal method using VOSO4 and NH3·H2O as precursors; the prepared material was used as a high performance cathode in LIBs.26
Much effort has been put into developing synthetic strategies for the fabrication of VO2 nanostructures, using methods such as sol–gel, templating, ion implantation, precursor pyrolysis, hydrothermal and solvothermal syntheses, magnetron sputtering and gas-phase deposition.27–31 These methods generally produced VO2(R) or VO2(B), but the metastable VO2(A) was only obtained by the hydrothermal method. Therefore, hydrothermal synthesis is considered to be the most favorable and flexible method for the synthesis of phase pure VO2(A) as well as being scalable, simple in concept, and environmentally benign. However, hydrothermal synthesis can be very complex mechanistically, as several metastable and stable hydrates and nonhydrate oxides may form upon rapid heating to high temperature in a pressurized autoclave.32 The abundance of phases and complexity of the hydrothermal phase diagram make it difficult for the formation and stabilization of each specific phase under specified conditions. It is not surprising that VO2(A) was often reported as the intermediate phase during transition from the metastable monoclinic VO2(B) to the more stable tetragonal rutile VO2(R) phase, with changes in temperature, pressure, and time. This leads to an ambiguous understanding of the transformation between VO2 polymorphs and therefore, the formation mechanism of VO2(A).25,33,34 Hence, it is still a great challenge to synthesize phase pure VO2(A).
In this paper, we have investigated the facile synthesis of VO2(A) monocrystalline nanoplates via a one-step hydrothermal process by the hydrolysis of a high concentrated (2.0 M) VO(acac)2 solution. The formation mechanism of the nanoplates has been characterized by both ex situ characterization and in situ powder X-ray diffraction (PXRD). In addition, the activity of the nanoplates as a novel biomimetic catalyst for the oxidation of the substrate 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB) in the presence of H2O2 has been determined.
2. Experimental section
2.1 Materials
Vanadyl acetylacetonate (VO(acac)2), sodium acetate (CH3COONa), H2O2 (30 wt%), and 3,3,5,5-tetramethylbenzidine dihydrochloride (TMB) were purchased from Aladin Ltd. (Shanghai, China). All chemicals used in this study were commercially available analytical grade and used without further purification.2.2 Synthesis
VO2(A) nanoplates were synthesized by a facile hydrothermal method using VO(acac)2 as the vanadium source. In a typical synthesis, 30 mmol (7.96 g) of VO(acac)2 was dispersed in 15 mL deionized water and then the mixed suspension was transferred into a 25 mL PTFE-sealed autoclave. The autoclave was heated to 220 °C and kept at that temperature for 24 h before it was cooled to room temperature in air. The resulting precipitate was filtered, washed with distilled water and ethanol three times, and finally dried at 60 °C under vacuum overnight.2.3 Ex situ characterization
The PXRD patterns were recorded on a Rigaku D/Max-RB X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å). A small amount of products were ultrasonically dispersed in ethanol and deposited on copper grids for transmission electron microscopy (TEM, JEM-2010F, JEOL) measurements. Field emission scanning electron microscopy (FESEM, JSM-6700F, JEOL) was employed to examine the morphology of the products. X-ray photoelectron spectroscopy (XPS) spectra were obtained on a VG ESCALAB MK II system equipped with Al KR radiation as the X-ray source. The measurements were carried out in an ultrahigh vacuum (UHV) chamber. The carbonaceous C1s line (284.5 eV) was used as the reference for the calibration of the binding energies.2.4 In situ PXRD
One in situ PXRD experiment was conducted on the powder diffraction beamline at the Australian Synchrotron and two laboratory-based in situ PXRD experiments were carried out using an Inel EQUINOX 3000 instrument at CSIRO Mineral Resources Flagship. For the synchrotron-based in situ PXRD experiment, the X-ray wavelength (0.6889 Å) was calibrated using a LaB6 standard (NIST SRM 660b). For the laboratory-based in situ PXRD, a Mo Kα radiation (λ = 0.7093 Å) was used. The same sample presentation setup was used in both synchrotron- and laboratory-based in situ experiments and was described in detail elsewhere.35–37 The starting precursor (5.4 mg VO(acac)2 and 10.7 μL Milli-Q water) was injected into a quartz glass capillary (1 mm in diameter, 0.1 mm in wall thickness, and 40 mm in length), which was then fitted to a custom-made stainless steel holder. The precursor-containing capillary/stainless steel holder was then attached to the goniometer head of the diffractometer and heated to the synthesis temperature 220 °C by a hot air blower beneath the capillary. External N2 pressure (3 MPa) was applied to the capillary during the synthesis to prevent vaporization of the solvent. For the synchrotron-based in situ experiments, the heating rate was 5 °C min−1, which is similar to the heating rate of a laboratory scale autoclave. For the lab-based in situ experiments, faster (30 °C min−1) and slower (1 °C min−1) heating rates were used to study the effect of heating rate on the syntheses mechanism. The temperature was monitored by a K-type thermocouple 3.5 mm beneath the capillary. In situ PXRD patterns were collected simultaneously during the synthesis with a time resolution of ∼2 min. The capillary was oscillated continuously during the measurements, which ensured temperature homogeneity and minimized potential effects of preferred orientation. For the lab-based in situ experiments, a surveillance camera was also used for monitoring visual appearance of the contents of the capillary reaction vessel as the synthesis reactions progressed.2.5 Peroxidase-like activity of the as-synthesized VO2(A) nanoplates
The peroxidase-like activity of VO2(A) nanoplates was evaluated as follows: 30 μL VO2(A) dispersion (0.3 mg mL−1) was mixed with 3 mL CH3COONa buffer solution (0.1 M, pH 4.0) containing 100 μM TMB and 5 mM H2O2. The mixture was then measured in wavelength-scan mode or time-scan mode by monitoring the absorbance change of TMB at 652 nm for 600 s on a UH4150 UV-visible spectrophotometer at 25 °C.The peroxidase-like reaction,38 catalyzed by VO2(A) nanoplates, can be described as follows:
The kinetic data were obtained under the optimum conditions by changing the concentrations of TMB and keeping the concentrations of H2O2 constant, or vice versa. The apparent steady-state reaction rates of the VO2(A) nanoplates were deduced according to their absorbance data and the molar absorption coefficient of TMB-derived oxidation products (ε = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1). The dynamics parameters (Vmax and Km) were determined using the Michaelis–Menten equation and a Lineweaver–Burk plot:
000 M−1 cm−1). The dynamics parameters (Vmax and Km) were determined using the Michaelis–Menten equation and a Lineweaver–Burk plot:
3. Results and discussion
3.1 Structure and morphology
Single crystalline tetragonal VO2(A) phase nanoplates were synthesized on a large scale under mild hydrothermal condition by the hydrolysis of a 2.0 M VO(acac)2 solution at 220 °C for 24 h. The phase identity of the product was confirmed by PXRD (Fig. 1), where the reflections match well with those of metastable tetragonal VO2(A) (space group: P4/ncc, ICDD database no. 70-2716). The absence of any other phases suggests the synthesis of pure VO2(A) phase. The chemical state and the compositions of the VO2(A) nanoplates were also confirmed by XPS, as shown in Fig. S1.† | ||
| Fig. 1 Ex situ powder X-ray diffraction pattern collected for the VO2(A) nanoplates prepared at 220 °C for 24 h. | ||
The morphology of the VO2(A) nanoplates can be observed in the TEM and SEM images shown in Fig. 2. Fig. 2a displays a representative SEM image of the high yield VO2(A) materials. The high-magnification SEM image (Fig. 2b) distinctly shows that the VO2(A) nanostructures are composed of uniformly plate-like nanoparticles with average width measuring ∼30–50 nm, length of 100–200 nm and thickness of 20–30 nm, respectively. The TEM image shown in Fig. 2c also confirms the plate-like morphology which is typically 20–40 nm wide and 100–200 nm long, in agreement with SEM observation. The high-resolution TEM (HRTEM) image (Fig. 2d) demonstrates the well-resolved lattice fringes with an interlayer spacing of about 0.60 nm, corresponding to the (110) lattice plane of VO2(A). Selected-area electron diffraction (SAED) pattern (inset of Fig. 2c) further validates the single crystalline nature of a single nanoplate.
3.2 Ex situ characterization of the samples synthesized under different conditions
In order to investigate the growth processes of VO2(A) tetragonal crystalline nanoplates, a series of experiments were conducted to track the evolution of morphologies and phases as a function of temperature and time by FESEM and PXRD. Temperature played an important role in controlling the size, shape, and crystal structures of the nanoparticles. By varying the hydrothermal synthesis temperature, the nanoparticles exhibited distinctly different shapes and morphologies, which is consistent with the evolution of crystalline structures. The PXRD patterns collected for the samples hydrothermally treated at 140 °C match well with pure V2O4·2H2O39 (ICDD database no. 13-0346) (Fig. 3a). All the peaks were the characteristic structures of layered hydrates of V2O4·2H2O particles.40 This phase was found to be stable when temperature was increased to 160 °C. Their solution synthesis and thermal stability have been previously fully analyzed by our group.39 Bunches of microrods (1–8 μm in length and 0.5–1 μm in diameter) consisting of tiny nanoparticle nuclei have been observed at 140 and 160 °C, as shown in the insets of Fig. 4a and b, respectively. As the temperature increased to 170 °C, the PXRD pattern shows the coexistence of V2O4·2H2O and VO2(A) (Fig. 3c). Correspondingly, some sheet-like VO2(A) nanoparticles were produced by consuming surrounding V2O4·2H2O microrods (Fig. 4c). However, when the temperature was further increased up to 180 °C, VO2(A) started to form slowly (Fig. 3d). The intensities of the VO2(A) phase reflections increase continuously with elevated reaction temperatures in Fig. 3. The crystalline sizes were estimated from the (110) diffraction peak at 2θ = 14.7° using the Scherrer formula. They were calculated to be 15.0 nm for 180 °C-24 h sample, 13.2 nm for 200 °C-10 h sample, 14.8 nm for 200 °C-24 h sample and 19.2 nm for 220 °C-24 h sample, respectively. At the same time, irregular nanosheets stacked randomly and exhibited a well-dispersed morphology (Fig. 4d–f). VO2(A) ordered nanoplates formed only when the temperature was raised to 220 °C (Fig. 4g), indicating that higher reaction temperature is beneficial for increasing crystallization rate and for uniformity of morphology of the particles.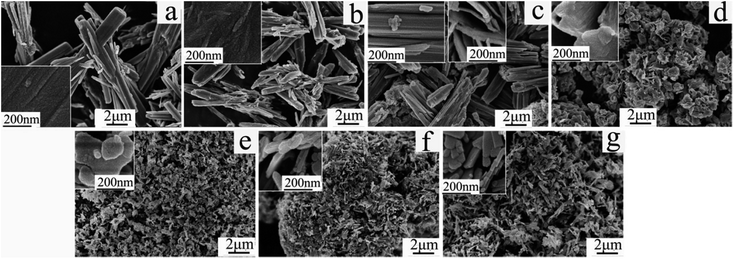 | ||
| Fig. 4 SEM images of the samples synthesized at different temperatures for 24 h. (a) 140 °C, (b) 160 °C, (c) 170 °C, (d) 180 °C, (e) 200 °C-10 h, (f) 200 °C, (g) 220 °C. | ||
To elucidate in depth the formation mechanism of the plate-like VO2(A) architecture, time-dependent hydrothermal synthesis experiments were carried out at 220 °C. The ex situ PXRD patterns of the phase evolution of the nanocrystals as a function of time are presented in Fig. 5 and S2a.† All of the reflections match well with the ICDD pattern for pure VO2(A) with tetragonal symmetry (no. 70-2716), and no intermediate phases or impurities were observed. The absence of intermediate phases will be further verified by in situ PXRD experiments (to be discussed later). We also observed that the phase abundance and crystallite size increase with increasing synthesis time from 1 to 24 h, as the peaks became higher and narrower with time. The crystalline sizes estimated from the (110) diffraction peak are 9.5 nm, 12.5 nm, 14.3 nm, 14.5 nm, 16.2 nm and 19.2 nm for the reactions carried out for 1, 3, 6, 10, 18 and 24 h, respectively.
When the synthesis time exceeds 30 min, single phase of VO2(A) nanosheets assembled from nanowires were obtained (as shown in Fig. S2†). After synthesis for 1 h and 3 h, 3D urchin-like microspheres having a diameter of ∼2–5 μm were formed (Fig. 6a and c). The hierarchical urchin-like superstructures are composed of radially aligned nanobelts having a thickness of ∼30 nm, width in the range of 100–200 nm and length up to 100 nm (Fig. 6b and d). More interestingly, exfoliation from the surface of the layer associated with splitting to narrow plates can be clearly observed (highlighted by red circles in Fig. 6b). It is likely that the existence of intrinsic stress and surface energy caused the rolling up of the nanosheets and subsequent exfoliation and splitting. When the reaction time was prolonged to 10 h and above, the sheets were cleaved into well aligned plate-shaped building blocks, as demonstrated in Fig. 6f–h.
 | ||
| Fig. 6 SEM images of samples synthesized hydrothermally at 220 °C for various reaction times. (a and b) 1 h, (c and d) 3 h, (e) 6 h, (f) 10 h, (g) 18 h, (h) 24 h. | ||
3.3 In situ characterization of the formation of VO2(A) phase
The poor understanding of most hydrothermal syntheses is largely due to the challenge of carrying out in situ and real time characterization, because the high temperature and high pressure autoclave condition is not accessible to most characterization techniques. By application of the in situ PXRD technique for tracking hydrothermal and solvothermal syntheses, we have the opportunity of unveiling the ‘black box’ of hydrothermal syntheses and elucidating unambiguous synthesis mechanisms, similar to which has been achieved in recent studies.35–37,41 Fig. 7 shows the results of the synchrotron-based in situ PXRD experiments, with the accumulated time-resolved PXRD patterns viewed down the intensity axis. In this synthesis, a heating rate of 5 °C min−1 was used, very close to the heating scheme of laboratory autoclaves. It is clearly shown that hydrolysis of VO(acac)2 occurred at around 170 °C as all diffraction peaks of VO(acac)2 disappeared. Immediately following this, diffraction peaks of this phase appear at the same time there was a sharp increase in the intensity of small angle X-ray scattering (SAXS) tail at low angle below 1.5°. The appearance of the SAXS tail was most likely due to the formation of a great number of nuclei of VO2(A). Subsequently, the intensity of the VO2(A) diffraction peaks increased with time while the intensity of the SAXS tail dropped. The dropping of the SAXS tail suggests the moving of scattering to lower angles which means the formation of larger crystals. This is in agreement with the increasing sharpness of the diffraction peaks. However, a complete understanding of the SAXS feature requires a separate study using a dedicated high resolution SAXS instrument which is capable of covering of much wider q range. No other phases were observed. Hence, the synthesis of VO2(A) follows a direct crystallization mechanism, in which VO2(A) nucleates and subsequent grows from a supersaturated solution formed by hydrolysis of VO(acac)2.To study the effect of heating rate on the synthesis mechanism, another two laboratory-based in situ PXRD experiments were carried out using a heating rate of 1 °C min−1 (slow heating) and 30 °C min−1 (fast heating). The results are shown in Fig. S3–S5.† It was found that varying heating rate had no effect on the synthesis mechanism. VO2(A) nucleated and grew directly from solution following the complete hydrolysis of VO(acac)2 with no other intermediate phases observed. However, the laboratory in situ PXRD experiments provided more information from the colour change of the reaction mixture observed by the surveillance camera (Fig. S4†). For the slow heating experiment, the starting greenish blue precursor started to turn dark at 99 °C, becoming darker until turning black at 205 °C (Fig. S4†). This phenomenon suggests that VO2(A) nucleation may commence below 170 °C with the minor amount and poor crystalline nature of the nuclei possibly explaining why they were not detected using PXRD. These nucleation events were not detected by in situ PXRD though possibly due to the minor amount and poor crystalline nature of the nuclei.
In previous studies,25,42,43 VO2(B) was regarded as the inevitable intermediate product in the initial stage of hydrolysis of VO(acac)2 under mild hydrothermal condition. Usually, its formation was assigned to the condensation of [VO(H2O)5]2+ complex ions and subsequent formation of vertex and edge sharing VO6 octahedra. However, this intermediate VO2(B) phase was not observed in the in situ PXRD experiments performed here. This may be because the precursor concentration (2.0 M) used here was dozens of times higher than the precursors used in previous studies. The hydrolysis of the higher concentration precursor resulted in a very high concentration of dissolved vanadium species (e.g., [VO(H2O)5]2+) that directly triggered nucleation and subsequent growth of the thermodynamically more stable phase VO2(A) rather than the metastable VO2(B) phase.25 This is in agreement with the selective formation of VO2 polymorphs being greatly dependent on synthesis parameters including pressure, temperature, pH value, and precursor concentrations.23
Based on the above results, the formation mechanism of plate-shaped VO2(A) can be described as follows: (a) VO(acac)2 hydrolyzes to form [VO(H2O)5]2+ complex ions and then condenses to vertex and edge sharing VO6 octahedra; (b) direct nucleation of VO2(A) and growth of nanosheets with a layered structure; (c) plate-like nanostructures formation due to the exfoliation and splitting of the nanosheets in order to reduce system energy. Scheme 1 summarizes the hydrating–exfoliating–splitting process during the formation of the nanoplates. This mechanism was also proposed to elucidate the formation of other nanostructures. For example, it is observed in diverse cases such as the exfoliation formation of vanadium oxide (VO2(B),44 V2O4·2H2O,39 V2O4·0.25H2O,40 V3O7·H2O45), TiO2,46 MnO2,47 and Ag nanowires.48
3.4 Peroxidase-like activity
To investigate the peroxidase-like activity of the as-prepared VO2(A) nanoplates, experiments of catalytic oxidation of chromogenic substrate TMB were conducted in the presence or absence of H2O2. The peroxidase-like activity was evaluated based on the intensity of the absorbance peak centered at 652 nm produced by the blue oxidation product (oxTMB). In the control experiments, mixtures of TMB + VO2 and H2O2 + VO2 only showed negligible adsorption, and mixture of TMB + H2O2 showed no absorptions in the scan range 400–800 nm (Fig. 8a). By contrast, after adding VO2(A) into the TMB + H2O2 solution, the clear solution turned to blue and meanwhile a strong absorption peak at 652 nm were observed (Fig. 8a). These observations clearly confirm the outstanding peroxidase-like catalytic activity of VO2(A) nanoplates toward the oxidation reaction between TMB and H2O2. Similar to the natural enzyme (horseradish peroxidase, HRP) and most artificial nanozymes, catalytic oxidation of TMB by VO2(A) nanoplates is highly dependent on the concentration of VO2(A) and pH, as shown in Fig. 8b–d. Therefore, the subsequent experiments were performed at the optimal VO2 concentration of 3.0 μg mL−1 and at pH 4.0.The apparent kinetic parameters (the Michaelis constant Km and the maximal reaction velocity Vmax) were calculated based on a series of experiments carried out at 25 °C by varying one substrate concentration each time and keeping the other constant. Typical Michaelis–Menten curves were obtained as shown in Fig. S6.† The Km and Vmax parameters were obtained using Lineweaver–Burk double reciprocal plot and their comparison with other nano-inorganic peroxidase mimics is given in Table 1. Remarkably, the Km value (0.058 mM) of VO2(A) nanoplates with H2O2 as substrate is significantly lower than that of HRP (3.7 mM), and even much lower than other nanoenzyme mimics, suggesting that VO2(A) nanoplates has a higher affinity to H2O2 than HRP and other peroxidase mimics. The higher performance of VO2(A) may be attributed to the more “active site” on the surface of the VO2(A) nanoarchitecture when compared with HRP.38,55 In addition, Km of VO2(A) nanoplates with TMB as the substrate was about 50% lower than that of HRP, while being larger relative to some other nanoparticles enzyme mimics. This result indicates that a higher TMB concentration is required to achieve maximum activity of VO2(A) nanoplates.
| Catalyst | Km (mM) | Vmax (10−8 M s−1) | Ref. | ||
|---|---|---|---|---|---|
| TMB | H2O2 | TMB | H2O2 | ||
| VO2(A) nanoplates | 0.165 | 0.058 | 2.4 | 1.4 | This work |
| HRP | 0.434 | 3.7 | 10 | 8.7 | 49 |
| Ni(OH)2 NFs | 0.023 | 1.76 | 1.4 | 1.2 | 50 |
| NiO NFs | 0.018 | 1.77 | 2.4 | 2.1 | 50 |
| VO2(B) nanobelts | 0.146 | 1.69 | 131 | 177 | 51 |
| GO-COOH | 0.0237 ± 0.001 | 3.99 ± 0.67 | 3.45 ± 0.31 | 3.85 ± 0.22 | 52 |
| TiO2@CeOx | 0.30 ± 0.04 | 1.39 ± 0.15 | 12.0 ± 0.6 | 55 ± 5.0 | 53 |
| Sisal-like Co3O4 | 0.01513 | 0.8268 | 38 | ||
| rGO–CFs | 0.046 ± 0.001 | 14.72 ± 2.332 | 1.121 ± 0.007 | 21.71 ± 3.445 | 54 |
4. Conclusions
In summary, plate-like VO2(A) nanostructures were successfully synthesized by a facile, template-free and scalable hydrothermal synthesis. The VO2(A) nanoparticles consist of uniform nanoplates of 30–50 nm in width, 100–200 nm in length, and 20–30 nm in thickness. The formation of VO2(A) follows a direct crystallization mechanism as no intermediate phases were observed in both ex situ and in situ PXRD-based characterization. Based on the time-dependent experimental results, a hydrating–exfoliating–splitting model was proposed to describe the formation mechanism of the nanoplate structure. The produced VO2(A) materials showed outstanding peroxidase-like activity and may find potential applications in biotechnology.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (51172265), the Ministry of Science and Technology of China (2014AA032802), the Education Commission of Shanghai Municipal (14ZZ099), the Materials Genome Institute of Shanghai University (14DZ2261200) and the China Postdoctoral Science and Foundation (2014M561528). This research was partially undertaken on the powder diffraction beamline at the Australian Synchrotron, Victoria, Australia, through the Science and Industry Endowment Fund Special Research Program-Synchrotron Science. We thank Mr Jingchao Song for assistance with synchrotron data collection, and Dr Helen Brand and Dr Justin Kimpton for beamline setup. The CSIRO Office of the Chief Executive (OCE) Postdoctoral Fellowship is also acknowledged for financial support.Notes and references
- D. A. Davydov, A. I. Gusev and A. A. Rempel, JETP Lett., 2009, 89, 194–199 CrossRef CAS.
- Y. F. Sun, B. Y. Qu, S. S. Jiang, C. Z. Wu, B. C. Pan and Y. Xie, Nanoscale, 2011, 3, 2609–2614 RSC.
- G. Andersson, Acta Chem. Scand., 1954, 8, 1599–1606 CrossRef CAS.
- D. B. Mcwhan, M. Marezio, J. P. Remeika and P. D. Dernier, Phys. Rev. B: Solid State, 1974, 10, 490–495 CrossRef CAS.
- P. C. Liu, K. J. Zhu, Y. F. Gao, Q. L. Wu, J. S. Liu, J. H. Qiu, Q. L. Gu and H. J. Zheng, CrystEngComm, 2013, 15, 2753–2760 RSC.
- F. Theobald, R. Cabala and J. Bernard, J. Solid State Chem., 1976, 17, 431–438 CrossRef CAS.
- D. Hangrman, J. Zubieta, C. J. Warren and L. M. Meyer, J. Solid State Chem., 1998, 138, 178–182 CrossRef.
- L. Liu, F. Cao, T. Yao, Y. Xu, M. Zhou, B. Y. Qu, B. C. Pan, C. Z. Wu, S. Q. Wei and Y. Xie, New J. Chem., 2012, 36, 619–625 RSC.
- C. Z. Wu, Z. P. Hu, W. Wang, M. Zhang, J. L. Yang and Y. Xie, Chem. Commun., 2008, 3891–3893 RSC.
- M. K. Chine, F. Sediri and N. Gharbi, Mater. Sci. Appl., 2011, 2, 964–970 CAS.
- L. Soltane, F. Sediri and N. Gharbi, Mater. Res. Bull., 2012, 47, 1615–1620 CrossRef CAS PubMed.
- M. D. Wei, H. Sugihara, I. Honma, M. Ichihara and H. S. Zhou, Adv. Mater., 2005, 17, 2964–2969 CrossRef CAS PubMed.
- C. Z. Wu, J. Dai, X. D. Zhang, J. L. Yang and Y. Xie, J. Am. Chem. Soc., 2009, 131, 7218–7219 CrossRef CAS PubMed.
- Y. Xu, L. Zheng and Y. Xie, Dalton Trans., 2010, 10729–10738 RSC.
- C. Z. Wu, Y. Xie, L. Y. Lei, S. Q. Hu and C. Z. Yang, Adv. Mater., 2006, 18, 1727–1732 CrossRef CAS PubMed.
- C. Z. Wu, H. Wei, B. Ning, J. L. Yang and Y. Xie, Chem. Commun., 2010, 46, 1845–1847 RSC.
- Y. F. Gao, H. J. Luo, Z. T. Zhang, L. T. Kang, Z. Chen, J. Du, M. Kanehira and C. X. Cao, Nano Energy, 2012, 1, 221–246 CrossRef CAS PubMed.
- Y. F. Wu, L. L. Fan, W. F. Huang, S. M. Chen, S. Chen, F. H. Chen, C. W. Zou and Z. Y. Wu, Phys. Chem. Chem. Phys., 2014, 16, 17705–17714 RSC.
- C. Z. Wu and Y. Xie, Energy Environ. Sci., 2010, 3, 1191–1206 CAS.
- F. Théobald, J. Less-Common Met., 1977, 53, 55–71 CrossRef.
- Y. Oka, S. Sato, T. Yao and N. Yamamoto, J. Solid State Chem., 1998, 141, 595–598 Search PubMed.
- T. Yao, Y. Oka and N. A. Yamamoto, J. Solid State Chem., 1994, 112, 196–198 CrossRef CAS.
- S. D. Ji, F. Zhang and P. Jin, J. Solid State Chem., 2011, 184, 2285–2292 CrossRef CAS PubMed.
- M. Li, F. Y. Kong, L. Li, Y. X. Zhang, L. Chen, W. W. Yan and G. H. Li, Dalton Trans., 2011, 10961–10965 RSC.
- S. D. Zhang, B. Shang, J. L. Yang, W. S. Yan, S. Q. Wei and Y. Xie, Phys. Chem. Chem. Phys., 2011, 13, 15873–15881 RSC.
- L. Dai, Y. F. Gao, C. X. Cao, Z. Chen, H. J. Luo, M. Kanehira, J. Jin and Y. Liu, RSC Adv., 2012, 2, 5265–5270 RSC.
- Q. W. Shi, W. X. Huang, Y. X. Zhang, J. Z. Yan, Y. B. Zhang, M. Mao, Y. Zhang and M. Tu, ACS Appl. Mater. Interfaces, 2011, 3, 3523–3527 CAS.
- E. U. Donev, R. Lopez, L. C. Feldman and R. F. Haglund, Nano Lett., 2009, 9, 702–706 CrossRef CAS PubMed.
- B. S. Guiton, Q. Gu, A. L. Prieto, M. S. Gudiksen and H. Park, J. Am. Chem. Soc., 2005, 127, 498–499 CrossRef CAS PubMed.
- Z. F. Peng, W. Jiang and H. Liu, J. Phys. Chem. C, 2007, 111, 1119–1122 CAS.
- J. W. Hou, J. Z. Zhang, Z. P. Wang, Z. M. Zhang and Z. J. Ding, RSC Adv., 2014, 4, 18055–18060 RSC.
- P. Nørby, M. Ø. K. Jensen, N. Lock, M. Christensen and B. B. Iversen, RSC Adv., 2013, 3, 15368–15374 RSC.
- S. Miloševic, I. Stojkovic, S. Kurko, G. O. Jasmina and C. Nikola, Ceram. Int., 2012, 38, 2313–2317 CrossRef PubMed.
- S. D. Ji, F. Zhang and P. Jin, J. Phys. Chem. Solids, 2012, 73, 762–769 CrossRef CAS PubMed.
- J. Song, F. Xia, M. Zhao, Y. L. Zhong, W. Li, K. P. Loh, R. A. Caruso and Q. Bao, Chem. Mater., 2015, 27, 3471–3482 CrossRef CAS.
- F. Xia, D. Chen, N. V. Y. Scarlett, I. C. Madsen, D. Lau, M. Leoni, J. Ilavsky, H. E. A. Brand and R. A. Caruso, Chem. Mater., 2014, 26, 4563–4571 CrossRef CAS.
- W. Li, F. Xia, J. Qu, P. Li, D. Chen, Z. Chen, Y. Yu, Y. Lu, R. A. Caruso and W. Song, Nano Res., 2014, 7, 903–916 CrossRef CAS.
- Q. Wang, S. W. Liu, H. Y. Sun and Q. F. Lu, Ind. Eng. Chem. Res., 2014, 53, 7917–7922 CrossRef CAS.
- L. Y. Bai, Y. F. Gao, W. D. Li, H. J. Luo and P. Jin, J. Ceram. Soc. Jpn., 2008, 116, 395–399 CrossRef CAS.
- M. D. Wei, H. Sugihara, I. Honma, M. Ichihara and H. S. Zhou, Adv. Mater., 2005, 17, 2964–2969 CrossRef CAS PubMed.
- K. M. Ø. Jensen, C. Tyrsted, M. Bremholm and B. B. Iversen, ChemSusChem, 2014, 7, 1594–1611 CrossRef CAS PubMed.
- S. D. Zhang, Y. M. Li, C. Z. Wu, F. Zheng and Y. Xie, J. Phys. Chem. C, 2009, 113, 15058–15067 CAS.
- H. O. Zhu, C. Xiao, H. Cheng, F. Grote, X. D. Zhang, T. Yao, Z. Li, C. M. Wang, S. Q. Wei, Y. Lei and Y. Xie, Nat. Commun., 2014, 5, 3960 CAS.
- L. Liu, T. Yao, X. G. Tan, Q. H. Liu, Z. Q. Wang, D. C. Shen, Z. H. Sun, S. Q. Wei and Y. Xie, Small, 2012, 8, 3752–3756 CrossRef CAS PubMed.
- Y. F. Zhang, X. H. Liu, G. Y. Xie, L. Yu, S. P. Yi, M. J. Hu and C. Huang, Mater. Sci. Eng., B, 2010, 175, 164–171 CrossRef CAS PubMed.
- M. D. Wei, Y. Konishi, H. S. Zhou, H. Sugihara and H. Arakawa, Chem. Phys. Lett., 2004, 400, 231–234 CrossRef CAS PubMed.
- X. Wang and Y. D. Li, Chem.–Eur. J., 2003, 9, 300–306 CrossRef CAS PubMed.
- G. Viau, J. Y. Piquemal, M. Esparrica, D. Ung, N. Chakroune, F. Warmont and F. Fiévet, Chem. Commun., 2003, 2216–2217 RSC.
- C. Walkey, S. Das, S. Seal, J. Erlichman, K. Heckman, L. Ghibelli, E. Traversa, J. F. McGinnis and W. T. Self, Environ. Sci.: Nano, 2015, 2, 33–53 RSC.
- C. Ray, S. Dutta, S. Sarkar, R. Sahoo, A. Roy and T. Pal, J. Mater. Chem. B, 2014, 2, 6097–6105 RSC.
- G. D. Nie, L. Zhang, J. Y. Lei, L. Yang, Z. Zhang, X. F. Lu and C. Wang, J. Mater. Chem. A, 2014, 2, 2910–2914 CAS.
- Y. J. Song, K. G. Qu, C. Zhao, J. S. Ren and X. G. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- L. Artiglia, S. Agnoli, M. C. Paganini, M. Cattelan and G. Granozzi, ACS Appl. Mater. Interfaces, 2014, 6, 20130–20136 CAS.
- J. H. Hao, Z. Zhang, W. S. Yang, B. P. Lu, X. Ke, B. L. Zhang and J. L. Tang, J. Mater. Chem. A, 2013, 1, 4352–4357 CAS.
- R. André, F. Natálio, M. Humanes, J. Leppin, K. Heinze, R. Wever, H. C. Schröder, W. E. G. Müller and W. Tremel, Adv. Funct. Mater., 2011, 21, 501–509 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: XPS spectra of the samples, PXRD patterns and HRTEM images of the products obtained at 220 °C for 30 min, images captured by the surveillance camera at in situ experiment and details about enzyme assays (double reciprocal plots). See DOI: 10.1039/c5ra11014a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |








