Ultrasound-assisted removal of Al3+ ions and Alizarin red S by activated carbon engrafted with Ag nanoparticles: central composite design and genetic algorithm optimization
Abstract
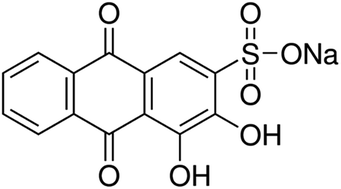
In this study, activated carbon engrafted with Ag nanoparticles (Ag-NPs–AC) was prepared and applied for ultrasonic assisted simultaneous removal of aluminum (Al3+) ions and Alizarin red S (ARS) dye from an aqueous medium. The physicochemical properties of Ag-NPs–AC were determined by different techniques such as SEM and FTIR. The effects of various operating parameters on the extent of adsorption were investigated. Optimum conditions were determined in order to achieve maximum removal percentage from the binary mixture. It was observed that the maximum performance for both the species was achieved at a pH of 6. The influence of different variables, such as the concentration of initial Al3+ (mg L−1) ions, ARS (mg L−1), adsorbent mass (mg) and sonication time (s), were studied using central composite design (CCD) combined with a desirability function approach (DFA) and genetic algorithm (GA). The isotherm models for the adsorption process were evaluated and the equilibrium data were accurately described by the Langmuir model with the maximum monolayer adsorption capacity for Al3+ ions and ARS as 222.2 and 232.6 mg g−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...