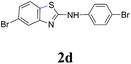Gold nanoparticle catalyzed intramolecular C–S bond formation/C–H bond functionalization/cyclization cascades†
Prasanta Gogoi*,
Bappi Paul,
Sukanya Hazarika and
Pranjit Barman
Department of Chemistry, National Institute of Technology, Silchar 788010, Assam, India. E-mail: prasantanits.11@gmail.com
First published on 23rd June 2015
Abstract
An efficient synthesis of 2-(N-aryl)aminobenzo[d]-1,3-thiazoles via intramolecular C–S bond formation/C–H bond functionalization utilizing an unusual cocatalytic Au-NPs/KMnO4 system under an oxygen atmosphere at 80 °C is presented. Au-NPs can be easily prepared by using HAuCl4 with reductive potential of Kayea assamica (sia nahor) aqueous fruit extract. The catalyst can be easily separated and recycled eight times without any appreciable loss of activity.
Introduction
The formation of C–S bonds is prevalent in the synthesis of a broad range of biologically active molecules and functional materials.1,2 In traditional methods, the cross-coupling of aryl halides with thiols/disulfides has become one of the dominant methods for the construction of C–S bonds.3–7 However, the pre-functionalization of the starting materials in this protocol limited its application. More recently, combination of selective, catalytic activation of C–H bonds with functionalization to form C–S bonds has been a subject of particular interest in recent years.8–10 Various catalytic systems based on copper,11 palladium,12–15 ruthenium16–19 and rhodium17–23 catalyzed C–S bond formation from thioamides via C–H functionalization has been realized. In this particular area, gold catalyzed intramolecular C–H functionalization has been less studied. Most of the systems involve a homogeneous process, and the problems associated with the removal of metal-residues from reaction products.24,25The use of Gold nanoparticles as catalysts inorganic reactions has attracted considerable interest as nanoparticles provide a larger number of active sites per unit area compared to their homogeneous counterparts. Furthermore, the nanomaterial catalyzed reactions provide the advantages of high atom efficiency, simplified isolation of product, and easy recovery and recyclability of the catalysts. Here we demonstrate a unique strategy for C–S bond construction through Au-NPs catalyzed oxidative C–H bond activation. To best of our knowledge, this is first example of Gold nanoparticles catalyzed intramolecular C–S bond formation via C–H bond functionalization.
Results and discussion
Synthesis of gold nanoparticles
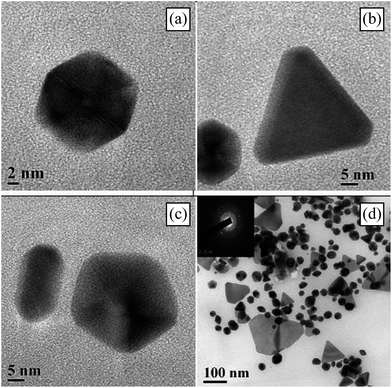
The use of plant systems has been measured as a green route and a consistent method for the biosynthesis of nanoparticles remaining to its environmental friendly nature.26,27 The Au-NPs were synthesized by using HAuCl4 with reductive potential of Kayea assamica, (K. assamica) King & Prain (Clusiaceae) aqueous fruit extract (Fig. 1, ESI†). K. assamica is a medicinally important plant. Its extract of flower (kayeassamins CI) has potential anticancer activity28 and the fruit of this species is used to kill fishes. The use of fruit extracts has more advantages than aqueous leaf extracts because later contains undesirable components like fats, chlorophyll and less polar compounds (Fig. 1).29In a typical procedure, 10 ml of fruit extract was added to 90 ml (10−3 M) aqueous solution of chloroauric acid and was stirred for 20 minutes. The progress of the reaction was routinely monitored by observing colour change as well as recording UV-visible spectrum (ESI†). The initial light-yellow solution turned to purple, indicating formation of colloidal gold. The supernatant containing Au-NPs was collected by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The solution was dried in a vacuum desiccator and the solid Au-NPs were collected for characterization. The XRD pattern of the prepared gold nanoparticles is shown in Fig. 2. The XRD pattern thus clearly shows that the gold nanoparticles were essentially crystalline. The result was consistent with TEM which shows a size distribution between 1 and 20 nm (Fig. 3). The five diffraction peaks at 38.1, 44.5, 64.8, 78.8 and 81.5° are indexed to the (111), (200), (220), (311) and (222) (JCPDS file, no. 89-3697) planes, respectively.
000 rpm. The solution was dried in a vacuum desiccator and the solid Au-NPs were collected for characterization. The XRD pattern of the prepared gold nanoparticles is shown in Fig. 2. The XRD pattern thus clearly shows that the gold nanoparticles were essentially crystalline. The result was consistent with TEM which shows a size distribution between 1 and 20 nm (Fig. 3). The five diffraction peaks at 38.1, 44.5, 64.8, 78.8 and 81.5° are indexed to the (111), (200), (220), (311) and (222) (JCPDS file, no. 89-3697) planes, respectively.
Catalytic activity
Initial optimization studies using model substrate 1a under catalytic Au-NPs usually not encouraging. Because typically Pd(0) afforded good activity.30,31 However, to standardize the reaction conditions, a series of experiments was performed with variation of different reaction parameters such as solvent, temperature, time and best results was obtained using 3 mol% Au-NPs in CH2Cl2 solvent in the presence of 10 mol% KMnO4 at 80 °C under an O2 atmosphere gave a 90% yield of 2a after 4 h (Table 1, entry 2).| Entry | Au-NPs (mol%) | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: diphenyl thiourea (1 mmol), Au-NPs (3 mol%), KMnO4 (10 mol%), in solvent (2.0 ml) under air for 4 h.b Isolated yield.c For 10 h argon atmosphere.d For 10 h nitrogen atmosphere.e Room temperature.f K2CO3 (1.5 mmol). | |||
| 1 | — | CH2Cl2 | Nr |
| 2 | 3 | CH2Cl2 | 90 |
| 3 | 5 | CH2Cl2 | 90 |
| 4 | 5 | DMF | 55 |
| 5 | 3 | H2O | 20 |
| 6 | 3 | THF | 30 |
| 7c | 3 | CH2Cl2 | 20 |
| 8d | 3 | CH2Cl2 | 10 |
| 9 | 5 | CH3CN | 45 |
| 10e | 3 | CH2Cl2 | 55 |
| 11f | 3 | CH2Cl2 | 87 |
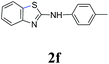
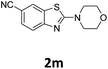
Under optimized reaction conditions (Table 2), we explored the substrate scope of this transformation. Arylthiourea containing methyl and methoxy groups on the aryl ring afforded desired product with excellent yields (2b, 2i). Para-cyano, -chloro, -bromo, and iodo groups were well-tolerated, furnishing amino benzothiazole derivatives in yields from 69% to 90%. When arylisothiocyanates (Table 2, 2k–m) were treated with morpholine, the resultant intermediate aryl thioureas (insitu) underwent the intramolecular heteroarylation reaction well in the presence of the catalyst. The strong electron donating group gives the maximum yield 95% (2l) while electron withdrawing group give least 75% (2k).
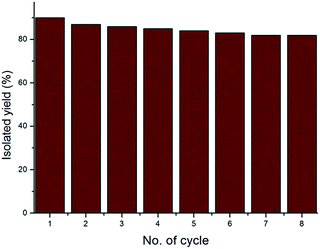
The mechanism of nanoparticle catalysed reactions is not easy to understand. However on the basis of previous studies,34 a catalytic cycle is proposed for the gold nanoparticle catalyzed intramolecular C–S bond formation via C–H bond functionalization (Fig. 5). In this scenario, thiourea 1 reacts with molecular oxygen in presence of catalytic amount of gold nanoparticles to form intermediate A. The intermediate A upon releasing H2O2 to gives intermediate B, which immediately gives the final product and regenerates the catalyst for the next catalytic cycle. The catalytic activity remains constant on addition of potassium permanganate. The catalyst was easily separated32,33 and was reused for 8th catalytic cycle without loss of its activity (Fig. 4).
To conclude, we report herein a simple method for the synthesis of Au-NPs by using K. assamica aqueous fruit extract solution. Au-NPs with size between 1–20 nm and different shapes were confirmed by TEM & XRD analysis. The present procedure using catalytic amount Au-NPs provides a very efficient and convenient methodology for the synthesis of 2-aminobenzothiazoles by oxidative C–H bond functionalization. The notable advantages offered by this procedure are operational simplicity.
Notes and references
- M. Safari, M. Yousefi, H. A. Jenkins, M. B. Torbati and A. Amanzadeh, Med. Chem. Res., 2013, 22, 5730–5738 CrossRef CAS.
- P. Chauhan, S. Mahajan and D. Enders, Chem. Rev., 2014, 114, 8807–8864 CrossRef CAS PubMed.
- W. Fu, T. Liu, Z. Fang, Y. Ma, X. Zheng, W. Wang, X. Ni, M. Hu and T. Tang, Chem. Commun., 2015, 51, 5890–5893 RSC.
- Y. Liu, B. Huang, X. Cao, D. Wu and J.-P. Wan, RSC Adv., 2014, 4, 37733–37737 RSC.
- Y. Liu, L. Zhou, X. Hui, Z. Dong, H. Zhu, Y. Shao and Y. Li, RSC Adv., 2014, 4, 48980–48985 RSC.
- P. Gogoi, S. Hazarika, M. J. Sharma, K. Sarma and P. Barman, Tetrahedron, 2014, 70, 7484–7489 CrossRef CAS PubMed.
- P. Gogoi, S. R. Gogoi, M. Kalita and P. Barman, Synlett, 2013, 24, 873–877 CrossRef CAS PubMed.
- C. Shen, P. Zhang, Q. Sun, S. Bai, T. A. Hor and X. Liu, Chem. Soc. Rev., 2015, 44, 291–314 RSC.
- J. Feng, G.-P. Lu and C. Cai, RSC Adv., 2014, 4, 54409–54415 RSC.
- S. R. Guo, Y. Q. Yuan and J. N. Xiang, Org. Lett., 2013, 15, 4654–4657 CrossRef CAS PubMed.
- X. Chen, X. S. Hao, C. E. Goodhue and J. Q. Yu, J. Am. Chem. Soc., 2006, 128, 6790–6791 CrossRef CAS PubMed.
- E. M. Beck, N. P. Grimster, R. Hatley and M. J. Gaunt, J. Am. Chem. Soc., 2006, 128, 2528–2529 CrossRef CAS PubMed.
- T. A. Dwight, N. R. Rue, D. Charyk, R. Josselyn and B. DeBoef, Org. Lett., 2007, 9, 3137–3139 CrossRef CAS PubMed.
- A. R. Dick, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2004, 126, 2300–2301 CrossRef CAS PubMed.
- T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed.
- V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 1731–1770 CrossRef CAS PubMed.
- P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS PubMed.
- L. Ackermann, L. Wang, R. Wolfram and A. V. Lygin, Org. Lett., 2012, 14, 728–731 CrossRef CAS PubMed.
- L. Ackermann and S. Fenner, Org. Lett., 2011, 13, 6548–6551 CrossRef CAS PubMed.
- D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2009, 110, 624–655 CrossRef PubMed.
- D. A. Colby, A. S. Tsai, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2011, 45, 814–825 CrossRef PubMed.
- A. S. Tsai, M. E. Tauchert, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2011, 133, 1248–1250 CrossRef CAS PubMed.
- G. Song, F. Wang and X. Li, Chem. Soc. Rev., 2012, 41, 3651–3678 RSC.
- Z. Shi and C. He, J. Org. Chem., 2004, 69, 3669–3671 CrossRef CAS PubMed.
- J. Xie, H. Li, J. Zhou, Y. Cheng and C. Zhu, Angew. Chem., 2012, 124, 1278–1281 CrossRef PubMed.
- S. Iravani, Green Chem., 2011, 13, 2638–2650 RSC.
- V. Kumar and S. K. Yadav, J. Chem. Technol. Biotechnol., 2009, 84, 151–157 CrossRef CAS PubMed.
- N. N. Win, S. Awale, H. Esumi, Y. Tezuka and S. Kadota, Bioorg. Med. Chem., 2008, 16, 8653–8660 CrossRef CAS PubMed.
- V. Kumar, S. C. Yadav and S. K. Yadav, J. Chem. Technol. Biotechnol., 2010, 85, 1301–1309 CrossRef CAS PubMed.
- X. Zhao, E. Dimitrijevic and V. M. Dong, J. Am. Chem. Soc., 2009, 131, 3466–3467 CrossRef CAS PubMed.
- L. L. Joyce and R. A. Batey, Org. Lett., 2009, 11, 2792–2795 CrossRef CAS PubMed.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- S. Praharaj, S. Nath, S. K. Ghosh, S. Kundu and T. Pal, Langmuir, 2004, 20, 9889–9892 CrossRef CAS PubMed.
- A. Monga and B. Pal, Part. Sci. Technol., 2015, 33, 159–165 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra10885c |
| This journal is © The Royal Society of Chemistry 2015 |