Syntheses, characterization, and magnetic properties of novel divalent Co/Ni coordination polymers based on a V-shaped pyridine ligand and dicarboxylate acids†
Abstract
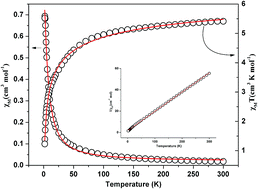
Solvothermal reactions of 3,5-bis(4-pyridyl)-pyridine (BPYPY) with 1,4-benzenedicarboxylate (1,4-bdc) and trans-1,4-cyclohexanedicarboxylic acid (trans-chdc) in the presence of Co(II) and Ni(II) salts in DMF/H2O or DMA/CH3CN/H2O produced three new compounds, namely, {[Co3(BPYPY)2(1,4-bdc)3(H2O)6]}n (1), {[Ni3(BPYPY)2(1,4-bdc)3(H2O)6]}n (2), {[Co(BPYPY)(trans-chdc)0.5(NO3)]·(H2O)}n (3). These compounds were characterized by elemental analysis, IR spectroscopy, and X-ray single-crystal diffraction. Compounds 1 and 2 are isomorphous and present 3,4-connected 3-fold interpenetrating tfz network with a point symbol of {63}2{64·8·10}3. Compound 3 exists binuclear Co clusters which are linked by BPYPY and trans-chdc to generate a 4-connected cds network with the point symbol of {65·8} and displays antiferromagnetic characteristics. In addition, the thermal stabilities of these new compounds have also been discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...