Hydrothermal synthesis of gold polyhedral nanocrystals by varying surfactant concentration and their LSPR and SERS properties†
Abstract
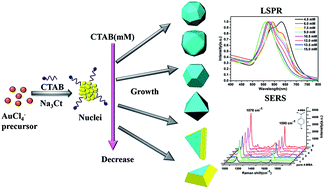
Several polyhedral gold nanocrystals (Au NCs) whose geometric morphology belongs to the Platonic solid and Archimedean solid groups are synthesized by modulating the cetyltrimethylammonium bromide (CTAB) concentration in the designed hydrothermal processes. The mechanism of growth of these polyhedral Au NCs is revealed via crystallographic analyses, which suggest that their morphologies mainly depend on the diminishing of the surface energy of the specific facets in the CTAB-directive shape-control process. Furthermore, the localized surface plasmon resonance (LSPR) properties of the as-prepared polyhedral Au NCs are numerically assessed using the finite element method (FEM). The calculations show that these polyhedral Au NCs exhibit geometry-dependence plasmonic properties: specifically, the enormous local field enhancements occur around their vertices and corners. The SERS spectra of 4-mercaptobenzoic acid molecules adsorbed on these polyhedral Au NCs display superior SERS activity with an enhancement factor of 105 to 106 at the excitation wavelength of 785 nm. Particularly, the truncated tetrahedron and bitetrahedron show higher SERS enhancement factors due to their LSPR being closer to the excitation wavelength of 785 nm. Thus, the facile surfactant-assisted synthesis offers the opportunity to design and optimize the shape-dependent SERS activity of polyhedral Au NCs with respect to possible applications in bio-sensing and imaging.

 Please wait while we load your content...
Please wait while we load your content...