Pickering emulsions stabilized by composite nanoparticles prepared from lysozyme and dopamine modified poly (γ-glutamic acid): effects of pH value on the stability of the emulsion and the activity of lysozyme
Cuige Zhangab,
Ye Zhua,
Rongli Zhangab,
Yanling Xiea,
Kangjing Wanga and
Xiaoya Liu*a
aKey Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China. E-mail: lxy@jiangnan.edu.cn; Fax: +86-510-85917763; Tel: +86-510-85917763
bSchool of Biochemical Engineering, Anhui Polytechnic University, Wuhu 241000, China
First published on 12th October 2015
Abstract
Novel bio-based composite nanoparticles were prepared through the electrostatic interaction between lysozyme (Lys) and amphiphilic dopamine-modified poly (γ-glutamic acid) (PGA-DA). The composite nanoparticles were used as particulate emulsifiers to stabilize white oil, forming gel-like emulsions. When the concentration of PGA-DA and Lys was 0.75 mg mL−1, the mean diameter of the composite nanoparticles was smallest (423 nm) with excellent stability. The influences of pH values on the properties of the composite nanoparticles, emulsifying performances and the activity of Lys in the emulsions were carefully investigated. Scanning electron microscopy (SEM) and laser scanning confocal microscopy (CLSM) characterization demonstrated that the composite nanoparticles adsorbed at the oil–water interface. Rheological measurements provided a possible stabilizing mechanism, which depended on the network structure of the adsorption layer at the interface-phase. Emulsifying properties were significantly improved with increasing the pH value of the solution. In addition, the bioactivity of emulsions at pH 6.2 was highest and well retained during a storage period of three months.
Introduction
Currently, Pickering emulsions have attracted tremendous interest in cosmetics, food, and pharmaceutical fields because of their potential applications.1 Many types of particulate emulsifiers can be used to stabilize Pickering emulsions, such as clays,2 silica,3 carbon nanotubes,4 nanocrystals,5 self-assembled nanoparticles,6–9 and microgels.10 Among these materials, self-assembled nanoparticles are one of the most promising candidates of particulate emulsifiers due to their controllable structure. Fujii11 reported shell cross-linked nano-aggregates self-assembled from poly{(ethylene oxide)-block-(glycerol monomethacrylate)-block-[2-(diethylamino) ethyl methacrylate]} (PEO–PGMA–PDEA) triblock copolymer and their applications as pH-responsive particulate emulsifiers for stabilization of 1-undecanol-in-water emulsions. Recently, poly{(glycerol monomethacrylate)-block-(2-hydroxypropyl methacrylate)-block-(ethylene glycol dimethacrylate)} (PGMA58–PHPMA350–PEGDMA20) cross-linked nanoparticles were used as particulate emulsifiers for stabilization of n-dodecane-in-water emulsions.12 Our group reported some nanoparticles self-assembled from amphiphilic copolymers for the stabilization of Pickering emulsions.13–15 However, most nanoparticles used to stabilize emulsions were prepared from synthetic polymers, which limited the applications in cosmetics, food and pharmaceuticals. Therefore, researchers have attempted to develop bio-based and environmentally friendly nanomaterials to stabilize emulsions, such as chitin nanocrystals,16 starch-based nanospheres,17 chitosan nanoparticles,18 ferritin19 and virus nanoparticles.20 Previously, our group reported the hyaluronic acid–chitosan (HA–CS) complex colloidal particles and their applications as biocompatible emulsifiers, the emulsion were well tolerated by the 3T3 cells, exhibiting low cytotoxicity. Furthermore, the HA–CS complex colloidal particles were used to load enzyme and stabilize Pickering emulsions, protecting the activity of enzyme.21 However, the pH value, which has important influence on emulsifying performances and the activity of enzyme in emulsions, has not been investigated.In our previous report, we studied the emulsifying performances of amphiphilic dopamine-modified γ-glutamic acid (PGA-DA) for white oil. The results revealed that PGA-DA with a random chain structure exhibited excellent emulsifying property above pH 5.0; however, PGA-DA nanoparticles had no emulsification below pH 5.0.22 Lysozyme (1,4-β-N-acetylmuramidase, Lys) is a lytic enzyme, which degrades the bacterial cell wall. Due to its bactericidal activity and thermal stability, Lys has been of interest in medicine, cosmetics, and food industry.23
In this work, Lys was added into amphiphilic PGA-DA solution, forming Lys@PGA-DA composite nanoparticle through an electrostatic interaction. The resulted composite nanoparticles were used as particulate emulsifiers to stabilize oil-in-water emulsions (Scheme 1). Herein, the influence of pH value on emulsifying performances and the activity of Lys in emulsions were carefully studied. In addition, the mechanism of emulsion stability was investigated.
 | ||
| Scheme 1 Schematic illustration of formation and oil-in-water interfacial behavior of Lys@PGA-DA composite nanoparticles. | ||
Experimental section
Materials
γ-PGA (sodium salt, Mw 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100
000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kDa) was purchased from AMRESCO, USA. Lysozyme and fluorescein isothiocyanate (FITC) were obtained from Sigma. Micrococcus lysodeikticus and coomassie brilliant blue (CBB) were obtained from Shanghai Sangon Company. White oil (26#), dopamine (DA) and azobisisoheptonitrile (ABVN) were obtained from Sinopharm Chemical Reagent Co., Ltd. Ultra-pure water was used for all the experiments. All materials were used without further purification. PGA-DA-28 (the modification degree of DA was 28%) polymers were synthesized according to the previously published method.24
000 kDa) was purchased from AMRESCO, USA. Lysozyme and fluorescein isothiocyanate (FITC) were obtained from Sigma. Micrococcus lysodeikticus and coomassie brilliant blue (CBB) were obtained from Shanghai Sangon Company. White oil (26#), dopamine (DA) and azobisisoheptonitrile (ABVN) were obtained from Sinopharm Chemical Reagent Co., Ltd. Ultra-pure water was used for all the experiments. All materials were used without further purification. PGA-DA-28 (the modification degree of DA was 28%) polymers were synthesized according to the previously published method.24
Preparation of Lys@PGA-DA composite nanoparticles
The aqueous solutions of Lys and PGA-DA were prepared with different initial concentrations from 0.5 mg mL−1, 1.0 mg mL−1, 1.5 mg mL−1 to 2 mg mL−1. By adding dropwise Lys solution to PGA-DA solution with equal volume and concentration under stirring, Lys@PGA-DA composite nanoparticles formed between negatively charged PGA-DA and positively charged Lys (pI ≈ 11).25 The final concentrations of Lys and PGA-DA in dispersions of composite nanoparticles were 0.25 mg mL−1, 0.5 mg mL−1, 0.75 mg mL−1 and 1 mg mL−1, respectively.Preparation of Pickering emulsion
White oil and composite nanoparticles aqueous solutions were mixed with equal volumes at room temperature (if no specific indication), the mixtures were homogenized at 8000 rpm for 2 min by a XHF-D H-speed dispersator homogenizer (1 cm head). The emulsions were sealed and placed under quiescence condition at room temperature after homogenization. The type of emulsions was determined by drop test, the test result showed that emulsions were oil in water type (O/W) emulsions.Activity determination of the emulsions
The emulsions were centrifuged at 8000 rpm for 20 min at 4 °C. After emulsions breaking, aqueous phase was extracted. According to Richard's method26 and definition of enzyme activity, Lys activity of aqueous phase was measured. The resulted activity was the activity of Lys in emulsions.Emulsion ratio measurements
The emulsion ratio was determined by measuring the volume of emulsion layer formed at the top of the bottle and total volume of emulsion sample.27 Creaming of emulsion samples was observed at room temperature.Characterization of Lys@PGA-DA composite nanoparticles
Dynamic light scattering (DLS) experiment was conducted using an ALV-5000/E dynamic light scattering instrument at 90°. Zeta potentials (ζ) of composite nanoparticles were conducted with a combination BIC 90Plus and Zeta PALS instrument (Brookhaven Instruments Corp., USA). The morphology of the composite nanoparticles was observed by the transmission electron microscope (TEM) using JEOL JEM-2100 at 200 kV.Characterization of emulsions
The morphology of emulsion droplets was observed with a digital optical microscopy (DM-BA450, Motic China Group Co., Ltd.) after a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dilution in the continuous-phase liquid. The particle size distribution and average size of emulsion droplets were executed using a Malvern MasterSizer 2000 (Malvern Instruments Ltd, Malvern, Worcestershire, UK). Ultra-pure water was used as the dispersant. The relative refractive index of the emulsion was taken as 1.27. Droplet size measurements were reported as the volume-average diameter. All determinations were conducted at least in duplicate. All tests were performed after a day of incubation.
6 dilution in the continuous-phase liquid. The particle size distribution and average size of emulsion droplets were executed using a Malvern MasterSizer 2000 (Malvern Instruments Ltd, Malvern, Worcestershire, UK). Ultra-pure water was used as the dispersant. The relative refractive index of the emulsion was taken as 1.27. Droplet size measurements were reported as the volume-average diameter. All determinations were conducted at least in duplicate. All tests were performed after a day of incubation.
An oil-phase solidification method14,18 was used to investigate the configuration of Lys@PGA-DA composite nanoparticles at oil–water interface. The powder after solidification was placed on electric conductive adhesive and sputter coated with thin layers of gold, and then observed with a Hitachi S-4800 field-emission microscope that was operated at an accelerating voltage of 20 kV.
The distribution of FITC-labeled Lys@PGA-DA in emulsion droplets interface was observed by confocal laser scanning microscopy (CLSM, Leica TCS SP8, Germany). The samples were excited by using a 488 nm He/Ne laser. A droplet of suspension was placed on a glass surface, and visualized directly.
Rheological measurements of the samples were performed by a discovery DHR-2 hybrid rheometer (TA Instruments, United States) equipped with a cone and plate geometry (25 mm cone diameter, 1.986°cone angle, 45 mm gap size) in a controlled shear-stress mode. Data were recorded with the Data Analysis equipment software Trios. Flow curves were determined using a steady-state flow ramp in the range of shear rate from 0.001 to 6000 s−1. The linear viscoelastic region (LVR) was assessed at 1 Hz by amplitude sweep experiments. Small deformation oscillatory measurements for evaluation of the viscoelastic properties, storage (elastic) modulus (G′, Pa) and loss (viscous) modulus (G′′, Pa) were performed over the frequency range of 0.1–10 Hz at 25 °C. All the rheological measurements were completed before any visual phase separation in the emulsions took place.
Results and discussion
Characterization of Lys@PGA-DA composite nanoparticles
The zeta potential and mean diameter of composite nanoparticles varying with polymer concentration are show as in Fig. 1A. As can be seen in curve a, the zeta potential of composite nanoparticles fluctuated around −47.5 mV. The reason can be ascribed to that the concentrations of Lys and PGA-DA were same in dispersions of Lys@PGA-DA composite nanoparticles. Curve b shows the mean diameter of composite nanoparticles with increasing the concentration of polymer. It can be seen that the diameter of composite nanoparticles significantly decreased when the concentration of PGA-DA and Lys increased from 0.25 mg mL−1 to 0.75 mg mL−1 (final concentrations, if no specific indication). When the concentration of PGA-DA and Lys increased to 1.0 mg mL−1, the diameter of nanoparticles slightly increased. As the concentrations were further increased, composite nanoparticles were precipitated due to larger particle size. The reason can be ascribed to that with increasing the concentration of PGA-DA and Lys from 0.25 mg mL−1, 0.5 mg mL−1 to 0.75 mg mL−1, the degree of interactions of lysozymes and PGA-DA increased (including electrostatic interactions, hydrogen bonding, hydrophobic interaction and van der Waals forces) because of the increase of concentrations and then formed a compact structure, leading to the decrease of particle size.28 When both concentrations increased to 1.0 mg mL−1, much more chains molecules aggregated, leading to the increase of particle size. However, the resulted composite nanoparticles are unstable to precipitate after 60 days. Considering to the stability of composite nanoparticles, the composite nanoparticles self-assembled from PGA-DA and Lys with concentration of 0.75 mg mL−1 were used to the following study.The Tyndall phenomenon, TEM image and the size distribution of composite nanoparticles are shown in Fig. 1B. As can be seen, the Lys@PGA-DA solution had obvious Tyndall phenomenon, indicating the formation of Lys@PGA-DA composite nanoparticles. The average diameter of composite nanoparticles estimated by DLS was 423 nm. The TEM image shows the uniformly spherical morphology with a size of 270 nm. The size of composite nanoparticles of TEM image is much smaller than that determined by DLS. The possible reason is that TEM reveals the diameter of dry particle, whereas dynamic light scattering analysis reveals the diameter of swelling particle in an aqueous solution.
Influence of pH value on emulsifying performances of Lys@PGA-DA composite nanoparticles
The pH value could affect the structure of composite nanoparticles, and further affect the emulsifying performances of composite nanoparticles. Therefore, the structure of composite nanoparticles was firstly studied before investigating emulsifying property varying with pH values. The zeta potential and diameter of composite nanoparticles were investigated in the pH range of 3.0–8.0. The result showed that the composite nanoparticles were unstable to flocculate due to less repulsion forces among the composite nanoparticles at pH 3.0; at pH 8.0, DA was easy to suffer from oxidation. In the pH range of 4.0–7.1, the zeta potential and diameter of composite nanoparticles with different pH values were showed in Fig. 2. As shown in curve a, the apparent zeta potential of composite nanoparticles decreased from −36.5 mV to −52 mV with increasing pH from 4.0 to 7.1. The possible reason is that with increasing pH values, deprotonation of the carboxylic acid group increases, resulting in the decrease of apparent zeta potential. Curve b shows the change trend of mean diameter of composite nanoparticles with different pH value. As can be seen, with increasing pH from 4.0 to 7.1, the size of composite nanoparticles increased from 212 nm to 545 nm. The reason can be ascribed to that with the increase of pH values, the decrease of apparent zeta potential results to the increase of electrostatic repulsion forces among the composite nanoparticles and the enhancement of hydrophilicity of composite nanoparticles, further leading to the formation of swelling composite nanoparticles and a dramatic increase in size. A similar swelling behavior of polymeric particles responding to pH changes was reported in the literature.29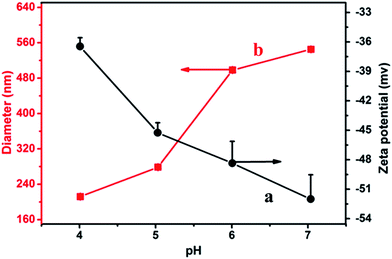 | ||
Fig. 2 Zeta potential (a) and mean diameter (b) of Lys@PGA-DA composite nanoparticles with different pH values. The concentration of PGA-DA and Lys was 0.75 mg mL−1 and WR was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Fig. 3 shows the emulsifying performances of Lys@PGA-DA composite nanoparticles with different pH values. As can be seen in Fig. 3A, with increasing pH value, the sizes of emulsion droplets gradually decreased. Fig. 3B shows the size distribution of emulsion droplets with different pH values. As can be seen, the size distributions of emulsion droplets with pH 5.1, pH 6.2 and pH 7.1 were single peak distributions except the sample with pH 4.0, which was a bimodal distribution. Fig. 3C (curve a) shows the mean sizes of emulsion droplets varying with the pH value. With increasing pH value from pH 4.0 to pH 7.1, the mean sizes of emulsion droplets gradually decreased from 122 μm to 68 μm. It may have been because with increasing pH value, the swollen degrees and deformability of composite nanoparticles increased, and then the oil–water interfacial area stabilized by composite nanoparticles was larger, resulting in a little droplet size and better emulsifying performance. In addition, with the increase of pH value, the emulsion ratios decreased initially and then slightly increased (Fig. 3C (curve b)). At pH 4.0, the emulsion ratio was highest, but the sizes of emulsion droplets was larger and the size distribution of emulsion droplets was a bimodal distribution, indicating the instability and slight flocculation of emulsions. The results indicated that the composite nanoparticles of moderate swelling exhibited a better emulsifying efficiency, as reported by previous literatrue.15
As direct evidence, the morphology of composite nanoparticles-coated droplets at oil–water interface was characterized by SEM and CLSM. As shown in Fig. 4, most of composite nanoparticles kept spherical structure at oil–water interface. Moreover, with increasing pH values, the deformation degree of composite nanoparticles gradually increased. The result further confirmed that the more deform the composite nanoparticles were, the larger interfacial area per composite nanoparticles occupied. CLSM image (Fig. 5) shows emulsion droplets with green fluorescence periphery, which is specific recognition of FITC for Lys, indicating the adsorption of composite nanoparticles at surface of oil drops and the formation of adsorption layer of composite nanoparticles. The adsorption layer can hinder the close approach of droplets, thus reducing the extent of coalescence.30
After 60 days of storage, the size of emulsion droplets with different pH values was showed in Fig. 6. As can be seen, in the pH range of 5.1–7.1, the mean size of emulsion droplets almost remained unchanged after 60 days of storage. The result suggested that the emulsion droplet had no flocculation during the process of storage, showing a good long-term stability. However, at pH 4.0, the mean size of emulsion droplets increased by about 40 μm compared to that of one day of preparation. Moreover, part of oil separated out, showing unstability of emulsions.
 | ||
| Fig. 6 The size of emulsion droplets stabilized by composite nanoparticles with different pH values, at one day of preparation and after 60 days of storage at ambient conditions. | ||
To further understand the mechanism of stabilization of emulsions, emulsions were characterized by rheological measurement.
Fig. 7A displays the flow curves of emulsions stabilized by composite nanoparticles with various pH values. As can be seen, with the increase of shear rates, emulsion viscosities gradually decreased and then reached the steady value, indicating a shear-thinning behavior. The shear-thinning behavior suggested the existence of weak associative interactions among the emulsion droplets and the formation of a weak droplet network structure. It also can be seen in Fig. 7A, the viscosity of the emulsions increased with increasing pH values at lower shear rates, which can be ascribed to that the increase of pH values resulted in the increase of swelling degree of composite nanoparticles, leading to the increase of expansion extent for hydrophilic chains of shell and the increase of emulsions viscosity.31 The previous studies reported that the change of structure of colloidal particles used as emulsion stabilizers can cause the change of viscosity in emulsion system.32,33 The result showed that the increase of system viscosities was important reasons of emulsion stability. Fig. 7B shows the rheological properties of emulsions characterized by dynamic oscillatory measurements. As can be seen, in all cases, the G′ slightly increased with the increase of oscillatory frequency in the linear viscoelastic range, indicating the formation of gel-like network.34 The reason can be ascribed to that the aggregate and crosslinking of Lys enhanced the interactions among composite nanoparticles containing Lys. In addition, with increasing pH value, the storage modulus (G′) and the loss modulus (G′′) increased. Moreover, G′ was higher than G′′ in the test linear viscoelastic rang and both were almost independent of frequency, indicating the formation of droplet network and predominantly elastic gel-like emulsions. A possible explanation for the gel-like emulsions formation could be composite nanoparticles interactions among the droplets. The interactions may come from the entanglement of chains of Lys and PGA-DA, which facilitated the formation of gel-like emulsions. Moreover, with increasing pH value, the interactions among the composite nanoparticles increased, resulting in further strengthen of droplet network and the stabilization of emulsions.
Rheological measurement of emulsions was performed after 60 days of storage. For the emulsions with pH 5.1, 6.2 and 7.1, the rheological properties were the same to emulsions of one day of preparation, indicating the stability of emulsions. However, the emulsions with pH 4.0 had no linear viscoelastic range, suggesting the un-stability of emulsions. The result was in agreement with the experimental result in Fig. 6. Additionally, the rheological experiments showed that with increasing pH values, the emulsions developed a viscoelastic structure which provided the emulsions with excellent long term stability to coalescence.
Influence of pH value on the activity of Lys in emulsions
Considering to practical application, the activity of Lys in emulsions was investigated. The change trend of activity with different pH values is shown in Fig. 8A. As seen, the value of activity increased initially and then decreased. The value of activity reached to the maximum at pH 6.2. The pH-activity curve of Lys was slightly shifted toward the alkaline pH range than for the curve of the native enzyme (the optimum pH 5.5). A similar shifting of the pH-activity curve has been observed.35 The result was explained that substrate–enzyme interactions affected the microenvironment in which an enzyme was embedded. Fig. 8B shows the activity of Lys in emulsions with time at pH 6.2. As can be seen, after 90 days incubation, the activity of Lys in emulsions decreased from 628 U mL−1 to 473 U mL−1 and retained the majority of activity, suggesting the Lys@PGA-DA composite nanoparticles provided an excellent matrix for Lys to retain its activity in emulsions. According to the literature,36 the contents of Lys in food industry are 0.005–0.6 mg mL−1 and the amount of Lys required to prevent late blowing of cheese is 500 U mL−1. In our study, the contents of Lys in nanoparticles are 0.25–1 mg mL−1 and the maximum value of activity is 628 U mL−1. Therefore, the emulsions system has a potential application prospect in food fields.Conclusions
In summary, we reported novel Lys@PGA-DA composite nanoparticles stabilized gel-like emulsion system. By introducing Lys to PGA-DA aqueous solution, the Lys@PGA-DA composite nanoparticles were obtained through the electrostatic interactions between positively charged Lys and negatively charged PGA-DA. After mixing vigorously with white oil, O/W gel-like emulsion formed. The increase of pH values facilitated crosslinks among composite nanoparticles and the formation of gel-like emulsions. Moreover, the emulsions retained the majority of bioactivity after a storage period of three months. Therefore, emulsions are a suitable delivery system and could be used in food fields.Acknowledgements
This work was supported by National Science Foundation of China (NSFC) number 20974041 and 21174056.References
- R. Aveyard, B. P. Binks and J. H. Clint, Adv. Colloid Interface Sci., 2003, 503, 100–102 Search PubMed.
- S. Abend and G. Lagaly, Clay Miner., 2001, 36, 557–570 CrossRef CAS PubMed.
- B. P. Binks, J. Philip and J. A. Rodrignes, Langmuir, 2005, 21, 3296–3302 CrossRef CAS PubMed.
- M. Shen and D. E. Resasco, Langmuir, 2009, 25, 10843–10851 CrossRef CAS PubMed.
- I. Kalashnikova, H. Bizot, B. Cathala and I. Capron, Langmuir, 2011, 27, 7471–7479 CrossRef CAS PubMed.
- X. Y. Liu, Y. H. Wang, C. L. Yi, Y. Feng, J. Q. Jiang, Z. G. Cui and M. Q. Chen, Acta Chim. Sin., 2009, 67, 447–452 CAS.
- C. L. Yi, J. H. Sun, W. Wei and X. Y. Liu, J. Funct. Polym., 2014, 27, 1–11 CAS.
- Y. Q. Yang, C. L. Yi, Y. H. Wang, J. Q. Jiang and X. Y. Liu, Acta Phys.-Chim. Sin., 2009, 25, 2225–2231 CrossRef CAS PubMed.
- J. Q. Wang, H. Y. Bai, C. L. Yi, N. Liu and X. Y. Liu, Acta Phys.-Chim. Sin., 2013, 29, 1028–1034 CAS.
- T. Ngai, H. Auweter and S. H. Behrens, Macromolecules, 2006, 39, 8171–8177 CrossRef CAS.
- S. Fujii, Y. Cai, J. V. M. Weaver and S. P. Armes, J. Am. Chem. Soc., 2005, 127, 7304–7305 CrossRef CAS PubMed.
- K. L. Thompson, P. Chambon, R. Verber and S. P. Armes, J. Am. Chem. Soc., 2012, 134, 12450–12453 CrossRef CAS PubMed.
- C. L. Yi, Y. Q. Yang, Y. Zhu, N. Liu, X. Y. Liu, J. Luo and M. Jiang, Langmuir, 2012, 28, 9211–9222 CrossRef CAS PubMed.
- X. Y. Liu, C. L. Yi, Y. Zhu, Y. Q. Yang, J. Q. Jiang, Z. G. Cui and M. Jiang, J. Colloid Interface Sci., 2010, 351, 315–322 CrossRef CAS PubMed.
- C. L. Yi, J. H. Sun, D. H. Zhao, Q. Hu, X. Y. Liu and M. Jiang, Langmuir, 2014, 30, 6669–6677 CrossRef CAS PubMed.
- M. V. Tzoumaki, T. Moschakis, V. Kiosseoglou and C. G. Biliaderis, Food Hydrocolloids, 2011, 25, 1521–1529 CrossRef CAS PubMed.
- Y. Tan, K. Xu, C. Liu, Y. L. Li, C. G. Lu and P. X. Wang, Carbohydr. Polym., 2012, 88, 1358–1363 CrossRef CAS PubMed.
- H. Liu, C. Y. Wang, S. W. Zou, Z. J. Wei and Z. Tong, Langmuir, 2012, 28, 11017–11024 CrossRef CAS PubMed.
- S. Fujii, A. Aichi, M. Muraoka, N. Kishimoto, K. Iwahori, Y. Nakamura and I. Yamashita, J. Colloid Interface Sci., 2009, 338, 222–228 CrossRef CAS PubMed.
- J. B. He, Z. W. Niu, R. Tangirala, J.-Y. Wang, X. Y. Wei, G. Kaur, Q. Wang, G. Jutz, A. Boker, B. Lee, S. V. Pingali, P. Thiyagarajan, T. Emrick and T. P. Russell, Langmuir, 2009, 25, 4979–4987 CrossRef CAS PubMed.
- D. H. Zhao, W. Wei, Y. Zhu, J. H. Sun, Q. Hu and X. Y. Liu, Macromol. Biosci., 2015, 15, 558–567 CrossRef CAS PubMed.
- R. L. Zhang, L. Lin, S. Xu, C. G. Zhang, X. Y. Liu and J. Luo, Colloids Surf., A, 2015, 470, 218–223 CrossRef CAS PubMed.
- D. Ercan and A. Demirci, Appl. Microbiol. Biotechnol., 2013, 97, 6211–6221 CrossRef CAS PubMed.
- R. L. Zhang, S. Xu, J. Luo, D. J. Shi, C. Liu and X. Y. Liu, RSC Adv., 2014, 4, 25106–25113 RSC.
- W. G. Burton, K. D. Nugent, T. K. Slattery, B. R. Summers and L. R. Snyder, J. Chromatogr. A, 1988, 443, 363–379 CrossRef CAS.
- R. M. Parry, R. C. Chandan and K. M. Shahani, Proc. Soc. Exp. Biol. Med., 1965, 119, 384–386 CrossRef CAS.
- L. Dokic, V. Krstonosic and I. Nikolic, Food Hydrocolloids, 2012, 29, 185–192 CrossRef CAS PubMed.
- M. Cegnar and J. Ker, Acta Chim. Slov., 2010, 57, 431–441 CAS.
- M. J. Snowden, B. Z. Chowdhry, B. Vincent and G. E. Morris, J. Chem. Soc., Faraday Trans., 1996, 92, 5013–5016 RSC.
- B. P. Binks, J. H. Clint and C. P. Whitby, Langmuir, 2005, 21, 5307–5316 CrossRef CAS PubMed.
- V. Michailova, S. t. Titeva, R. Kotsilkova, E. Krusteva and E. Minkov, Colloids Surf., A, 1999, 149, 515–520 CrossRef CAS.
- E. J. Stancik and G. G. Fuller, Langmuir, 2004, 20, 4805–4808 CrossRef CAS.
- N. P. Ashby, B. P. Binks and V. N. Paunov, Chem. Commun., 2004, 436–437 RSC.
- F. Liu and C. H. Tang, Food Chem., 2011, 127, 1641–1647 CrossRef CAS PubMed.
- A. D. McLaren and E. F. Estermann, Arch. Biochem. Biophys., 1957, 68, 157–160 CrossRef CAS.
- V. A. Proctor, F. E. Cunningham and D. Y. C. Fung, Crit. Rev. Food Sci. Nutr., 1988, 26, 359–395 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |






