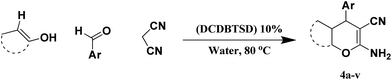
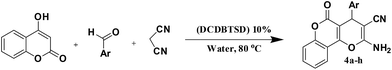
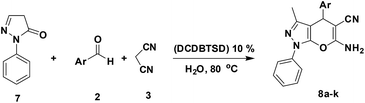
N,2-Dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide: an efficient and homogeneous catalyst for one-pot synthesis of 4H-pyran, pyranopyrazole and pyrazolo[1,2-b]phthalazine derivatives under aqueous media†
Ardeshir Khazaei*a,
Mohammad Ali Zolfigol*a,
Fatemeh Karimitabar a,
Iraj Nikokarb and
Ahmad Reza Moosavi-Zarec
a,
Iraj Nikokarb and
Ahmad Reza Moosavi-Zarec
aFaculty of Chemistry, Bu-Ali Sina University, Hamedan 6517838683, Iran. E-mail: Khzaei_1326@yahoo.com
bDepartment of Medical Biotechnology, Guilan University of Medical Sciences, Rasht, Iran
cSayyed Jamaleddin Asadabadi University, Asadabad, 6541835583, Iran
First published on 4th August 2015
Abstract
N,2-Dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide (DCDBTSD) as a highly efficient and homogeneous catalyst was successfully applied for the synthesis of 4H-pyran, pyranopyrazole and pyrazolo[1,2-b]phthalazine derivatives by a one-pot multi-component reaction (MCR) in water. The described method has some advantages such as mild and neutral reaction media, high yields, short reaction times, cleaner and easier reaction profiles and compliance with green chemistry protocols.
Introduction
Water, as a widely available, inexpensive, nonflammable, nonhazardous, nontoxic, and uniquely redox-stable solvent in enormous quantities, can accelerate the rate of organic reactions even for water-insoluble reactants as well as facilitate product isolation by straightforward filtration.1 Thus, there is a demand for the development of synthetically useful and convergent multicomponent reactions (MCRs) using water as a green reaction medium.1 Multi-component reactions (MCRs) play an important role in combinatorial chemistry because of their ability to prepare target compounds with greater efficiency and atomic economy by generating structural complexity in a single step from three or more reactants.2The preparation of tetrahydrobenzo[b]pyrans is important due to their significant anti-coagulant, diuretic, spasmolytic, anti-cancer, antihypertensive, calcium antagonistic, pharmaceutical and anti-anaphylactic properties.3 Several methods have been introduced for the synthesis of tetrahydro-4H-benzopyran derivatives using different catalysts such as [cmmim]Br,4a hexadecyldimethylbenzyl ammonium bromide (HDMBAB),4b Na2SeO4,4c [pyridine–SO3H]Cl,4d magnesium oxide,4e and (S)-proline.4f
Pyranopyrazoles are fused heterocyclic compounds, which are important because of their biological properties such as fungicidal,5a bactericidal,5b and vasodilatory activity;5c moreover, they act as anticancer agents.5d Some catalysts have been used to promote this reaction such as imidazole,6 [Dsim]AlCl4,7a silicotungstic acid,7b L-proline,7c and isonicotinic acid.7d
2H-Indazolo[2,1-b]phthalazine-triones, as N-heterocyclic compounds, show biological and pharmacological activities such as anticonvulsant, cardiotonic, and vasorelaxant.8 Various catalysts, including dodecylphosphonic acid,9a Ce(SO4)2,9b heteropolyacids,9c Mg(HSO4)2,9d silica sulfuric acid9e and p-TSA,9f have been used for the synthesis of these compounds.
However, some reported methods for the synthesis of the above-mentioned compounds suffer from one or more disadvantages, such as requiring toxic, corrosive, expensive and/or large amount of catalysts, having a long reaction time, requiring toxic and corrosive solvents and requiring strong acidic media. Because of the importance of these compounds, a search for a milder, more eco-friendly and faster method under green, neutral and aqueous conditions providing higher yields is still needed.
A large group of compounds generically called N-halo reagents are used as potentially reactive intermediates. These compounds are widely used in organic synthesis and in the chemistry of natural compounds. Some specific features of N-halo reagents, such as high activity of the N–X bond and the various modes of splitting of this bond, enable their wide application in organic transformations.10–12 Considering the abovementioned facts and in continuation of our previous studies on the applications of N-halo reagents in organic synthesis,13–21 we recently used N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide (DCDBTSD) (Fig. 1) as an efficient and homogeneous catalyst for the synthesis of 4H-pyran, pyrazolo[1,2-b] phthalazine and pyranopyrazole derivatives.
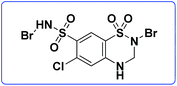 | ||
| Fig. 1 The structure of N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide (DCDBTSD). | ||
Results and discussion
N,2-Dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide (DCDBTSD) was prepared via a simple procedure and was fully characterized by IR, UV, 1H and 13C NMR, XRD, TG/DTG as well as mass spectrometry and was used as an efficient catalyst for the preparation of 4,40-(arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s via a one-pot pseudo five component condensation reaction of phenylhydrazine, acetoacetate derivatives and arylaldehydes.13To expand the application, the efficacy and the scope of DCDBTSD in the synthesis of heterocyclic compounds; initially, the synthesis of 4H-pyran derivatives via the one-pot condensation reaction of a reactive α-methylene group with aromatic aldehydes and malononitrile was studied.
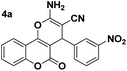
To optimize the reaction conditions, the reaction of 4-hydroxycoumarine (1) (1 mmol), 4-nitrobenzaldehyde (2) (1 mmol) and malononitrile (3) (1.2 mmol) as a model reaction was investigated with respect to the variation of reaction parameters such as catalyst quantity, reaction temperature and types of solvents. The results are summarized in Table 1.
| Entry | Amount of catalyst/mol% | Solvent | Temp. | Time (min) | Yieldb |
|---|---|---|---|---|---|
| a Model reactions: 4-hydroxycoumarine 1 (1 mmol), 4-nitrobenzaldehyde 2 (1 mmol), malononitrile 3 (1.2 mmol) and DCDBTSD.b Isolated yield. | |||||
| 1 | — | H2O | 80 | 240 | Trace |
| 2 | 5 | H2O | 80 | 70 | 82 |
| 3 | 10 | H2O | 80 | 30 | 88 |
| 4 | 20 | H2O | 80 | 30 | 88 |
| 5 | 10 | H2O | 60 | 60 | 79 |
| 6 | 10 | H2O | r.t | 150 | 40 |
| 7 | 10 | MeOH | 80 | 60 | 70 |
| 8 | 10 | EtOH | 80 | 40 | 85 |
| 9 | 10 | CH3CN | 80 | 60 | 78 |
| 10 | 10 | Neat | 80 | 60 | 68 |
As shown in Table 1, 10 mol% of DCDBTSD (0.0455 g) in water provided the best reaction conditions for the described reaction (Table 1, entry 3). Notably, no product was observed in the absence of catalyst (Table 1, entry 1), which further requires the use of DCDBTSD in this transformation. To optimize the reaction temperature, we also performed several experiments in water at 25, 60, and 80 °C. It was found that the best yield of product was achieved at 80 °C (Table 1, entry 3).
Moreover, the reaction could be efficiently performed in all the tested solvents (Table 1, entries 3 and 7–9). The reaction that used water as the solvent led to higher yields and shorter reaction time than those using methanol, ethanol and acetonitrile as solvent. Thus, water, which is abundantly available, low-cost, safe, and harmless, was chosen as the solvent for all further reactions.
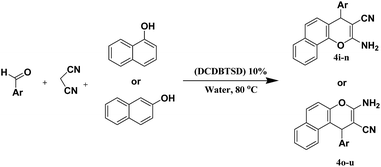
With the optimized conditions in hand, to outline this approach, the scope and generality of this protocol was next examined by employing a good range of aromatic aldehydes that possess a reactive α-methylene group and malononitrile (Scheme 1).
All the reactions proceeded efficiently under the optimized conditions (Tables 2 and 3).
| Entry | Product | Yielda | Time (min) | Mp (L)ref |
|---|---|---|---|---|
| a Isolated yield. | ||||
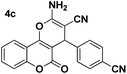
| 1 | 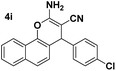 |
85 | 20 | 247–248 (247–248)24 |
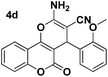
| 2 | 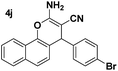 |
92 | 30 | 246–248 (243–244)24 |
| 3 | 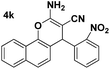 |
86 | 35 | 207–209 (207–209)24 |
| 4 | 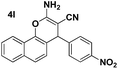 |
91 | 15 | 211–212 (213–214)24 |
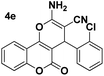
| 5 | 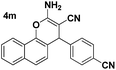 |
83 | 25 | 258–261 (259–260)25 |
| 6 | 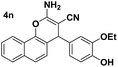 |
87 | 25 | 225–227 (222–223)24 |
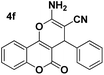
| 7 | 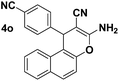 |
79 | 25 | 280–282 (284–286)25 |
| 8 | 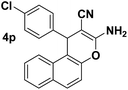 |
90 | 20 | 236–239 (237–238)26 |
| 9 | 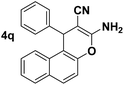 |
85 | 40 | 290–292 (291–292)13b |
| 10 | 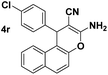 |
85 | 22 | 216–218 (214–215)24 |
| 11 | 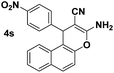 |
92 | 30 | 186–188 (187–189)27 |
| 12 | 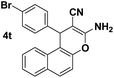 |
91 | 25 | 238–240 (238–240)27 |
| 13 | 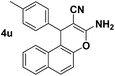 |
84 | 30 | 271–274 (273–275)14 |
Aromatic aldehydes bearing both electron-withdrawing and electron-donating groups in ortho, meta and para positions of the aromatic ring were converted into desired products in good to excellent yields.
Moreover, the presented methodology was used successfully for various carbonyl compounds that have a reactive α-methylene group, and corresponding desired products were obtained in good to excellent yields without any by-products being observed.
We also compared the result of the present DCDBTSD with other catalysts reported in the literature such as triethylbenzylammonium chloride (TEBA), PEI@Si–MNPs, hexamethylenetetramine (HMT) and TiO2 nanowires (TiO2 NWs) for the preparation of 4H-pyran derivatives (Table 4). Table 4 clearly demonstrates that DCDBTSD is an effective catalyst in terms of the reaction time and the yield of the obtained product is comparable to other reported catalysts.
| Entry | Catalyst | Conditions | Time (min) | Yielda | Ref. |
|---|---|---|---|---|---|
| a Yields refer to isolated pure products. Based on the reaction of 4-hydroxycoumarine 1 (1 mmol), 3-nitrobenzaldehyde 2 (1 mmol), malononitrile 3 (1 mmol) in corresponding conditions.b The present work. | |||||
| 1 | TEBA | H2O, 90 °C | 600 | 88 | 28 |
| 2 | PEI@Si–MNPs | H2O, reflux | 50 | 96 | 3 |
| 3 | HMT | EtOH, reflux | 40 | 95 | 23 |
| 4 | TiO2 NWs | EtOH/H2O (1/1), reflux | 40 | 90 | 29 |
| 5 | DCDBTSD | H2O, 80 °C | 20 | 95 | —b |
Indole holds a noticeable property among the various heterocyclic systems because it is present as a core unit in a number of compounds having a wide spectrum of biological activities.30
It has been reported that the spiro-oxindole heterocyclic framework is an important structural motif in biologically relevant compounds such as natural products and pharmaceuticals, e.g. surugatoxin, horsfiline, spirotryprostatin A and B, elacomine, gelsemine, alstonisine and strychnofoline.31–37
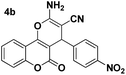
The development of efficient and convenient syntheses of novel bioactive organic compounds, such as spirooxindoles, is an important current research area, with MCRs considered to be the most efficient method of preparing spirooxindoles.38 To further expand the scope of the reaction, it was thought meaningful to replace aromatic aldehydes with N-alkyl isatin derivatives to show the versatility of this protocol (Scheme 2).
At first, N-alkyl isatin derivatives were prepared by the reaction of isatin with K2CO3 and alkyl halide (Scheme 3).39
To our surprise, N-alkyl isatin derivatives were easily transformed into the desired products in excellent yields (Table 5).
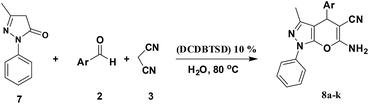
Heterocycles containing pyrazole rings are important target compounds in synthetic and medicinal chemistry because the ring is a key moiety in various biologically active compounds.38 Pyrazole derivatives have been widely studied in the development of insecticides, acaricides, fungicides, herbicides, dyes and other reagents because of their efficiency, low toxicity, unique reaction mechanisms, safety, lack of cross-resistance, and other characteristics.38,41 Being inspired by the abovementioned results and also in continuation of our interest in extending the scope of the N-bromo sulfonamide reagent in the synthesis of heterocyclic compounds, we investigated the three-component reaction of aromatic aldehydes possessing a 3-methyl-1-phenyl-2-pyrazolin-5-one and malononitrile in the presence of DCDMTSD (Scheme 3).
As can be seen from Table 6, for precursors bearing either electron-donating or electron-withdrawing groups, all the reactions proceeded very smoothly to provide the desired products. The electronic character of substituents on the aromatic ring of the aldehyde did not exert an obvious effect on the reaction yields. All the desired products were obtained in high yields and in short reaction times.
| Entry | Product | Yielda | Time (min) | Mp (L)ref |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | 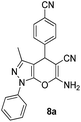 |
80 | 25 | 198–200 (197–199)42a |
| 2 | 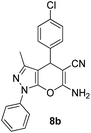 |
92 | 25 | 230–232 (233–235)42b |
| 3 | 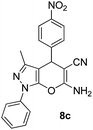 |
95 | 20 | 194–196 (192–195)42a |
| 4 | 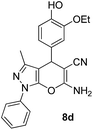 |
83 | 30 | 173–175 (170–171)43 |
| 5 | 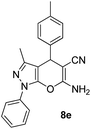 |
79 | 30 | 173–175 (172–174)29 |
| 6 | 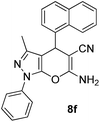 |
80 | 35 | 159–162 (159–160)43 |
| 7 | 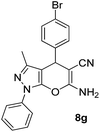 |
88 | 20 | 184–185 (184–185)42b |
| 8 | 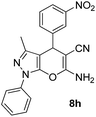 |
78 | 30 | 183–185 (188–191)29 |
| 9 | 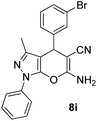 |
80 | 40 | 166–168 (169–170)30 |
| 10 | 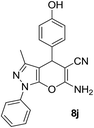 |
81 | 30 | 212–214 (210–212)29 |
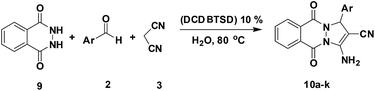
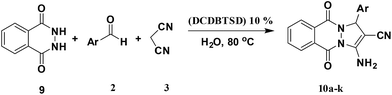
Heterocyclic compounds having bridgehead hydrazine groups have been studied for over a century owing to their pharmacological characteristics and clinical applications.38,44,45 Thus, numerous studies have been reported in the past five decades.46–50 To expand the application of DCDBTSD in the synthesis of heterocyclic compounds, we decided to prepare 3-amino-1H-pyrazolo[1,2-b]phthalazine-5,10-dione by a three-component reaction of aromatic aldehydes or N-alkyl isatin derivatives possessing a 2,3-dihydrophthalazine-1,4-dione and malononitrile (Scheme 4).
As shown in Table 7, the presented procedure provides an efficient and green approach for the synthesis of 3-amino-1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives. According to the obtained results (Table 7), DCDBTSD could be applicable for the synthesis of various types of nitrogen-containing heterocyclic compounds.
| Entry | Product | Yielda | Time (min) | Mp (L)ref |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | 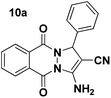 |
80 | 30 | 271–273 (276–278)38 |
| 2 | 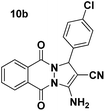 |
93 | 15 | 271–273 (270–272)51 |
| 3 | 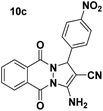 |
95 | 15 | 229–230 (228–229)52 |
| 4 | 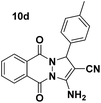 |
82 | 25 | 250–252 (253–255)38 |
| 5 | 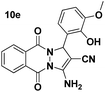 |
85 | 35 | 154–156 (152–154)52 |
| 6 | 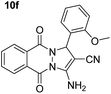 |
80 | 20 | 250–252 (248–250)53 |
| 7 | 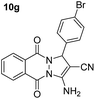 |
87 | 20 | 264–266 (263–265)53 |
| 8 | 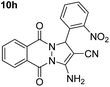 |
87 | 20 | 268–268 (266–267)53 |
| 9 | 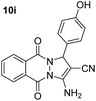 |
80 | 35 | 271–273 (270–272)54 |
| 10 | 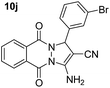 |
81 | 15 | 274–276 (271–273)53 |
| 11 | 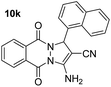 |
84 | 20 | 275–277 (276–278)51 |
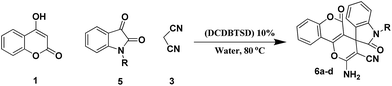
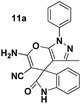
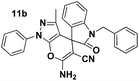
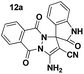
To evaluate the generality and versatility of our catalyst for the preparation of bioactive compounds, we decided to synthesise spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives and 3′-aminospiro[indoline-3,1′-pyrazolo[1,2-b]phthalazine]-2,5′,10′-trione using DCDBTSD (Scheme 5). For this purpose, we examined the reaction of N-substituted isatins, 3-methyl-1-phenyl-2-pyrazolin-5-one or 2,3-dihydrophthalazine-1,4-dione with malononitrile (Scheme 5).
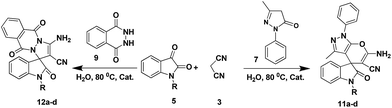 | ||
| Scheme 5 Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] (11a–d) and spiro[indoline-3,1′-pyrazolo[1,2-b]phthalazine]-2,5′,10′-trione (12a–d) derivatives in the presence of DCDBTSD. | ||
Tables 8 and 9 indicate that the desired products were obtained in all the cases with excellent yields. Our effort to use N-substituted isatins as starting materials with active carbonyl functional groups in the above mentioned MCR was also successful, verifying the flexibility of the existing procedure.
Experimental
Materials
All the chemicals were purchased from Merck or Fluka Chemical. The known products were identified by comparison of their physical properties, such as melting points and spectral data, with those reported in the literature.General procedure for the synthesis of DCDBTSD
A solution of sodium hydroxide (6 mol L−1, 1 mL) was added dropwise into a round bottomed flask (50 mL) containing hydrochlorothiazide (0.6 g, 2 mmol) in distilled water (2 mL) with constant stirring over a period of 10 min at room temperature. After the addition was completed, the reaction mixture was stirred for 20 min. Subsequently, to the stirring solution of hydrochlorothiazide, bromine (0.08 mL, 3 mmol) was slowly added over a period of 15 min at 0 °C. The insoluble brominated catalyst was removed by filtration and washed with H2O (10 mL) to obtain N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide (DCDBTSD) in 90% yield (0.82 g).13General procedure for the synthesis of 4a–u
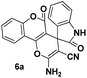
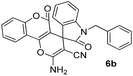
Carbonyl compounds possessing a reactive α-methylene group 1 (1 mmol), aromatic aldehyde 2 (1 mmol), malononitrile 3 (1.2 mmol) and DCDBTSD (0.0455 g, 10 mol%) were added to 2 mL water and the reaction mixture was stirred at 80 °C for the appropriate time, as mentioned in Tables 2 and 3. After the completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, the solid product was obtained by simple filtration and the solid residue was finally recrystallized from EtOH.General procedure for the synthesis of 6a–d
4-Hydroxy-2H-chromen-2-one 1 (1 mmol), isatin 5 (1 mmol), malononitrile 3 (1.2 mmol) and DCDBTSD (0.0455 g, 10 mol%) were added to 2 mL water and the reaction mixture was stirred at 80 °C for the appropriate time, as mentioned in Table 5. After the completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, the solid product was obtained by simple filtration and the solid residue was finally recrystallized from EtOH.General procedure for the synthesis of 8a–k
A mixture of aromatic aldehyde 2 (1 mmol), malononitrile 3 (1.2 mmol), 3-methyl-1-phenyl-2-pyrazolin-5-one 7 (1 mmol) and DCDBTSD (0.0455 g, 10 mol%) were added to 2 mL water and the reaction mixture was stirred at 80 °C for the appropriate time, as mentioned in Table 6. After the completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, the solid product was obtained by simple filtration and the solid residue was finally recrystallized from EtOH.General procedure for the synthesis of 10a–k
A mixture of aromatic aldehyde 2 (1 mmol), malononitrile 3 (1.2 mmol), 2,3-dihydrophthalazine-1,4-dione 9 (1 mmol) and DCDBTSD (0.0455 g, 10 mol%) were added to 2 mL water and the reaction mixture was stirred at 80 °C for the appropriate time, as mentioned in Table 7. After the completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, the solid product was obtained by simple filtration and the solid residue was finally recrystallized from EtOH.General procedure for the synthesis of 11a–d
A mixture of isatin 5 (1 mmol), malononitrile 3 (1.2 mmol), 3-methyl-1-phenyl-2-pyrazolin-5-one 7 (1 mmol) and DCDBTSD (0.0455 g, 10 mol%) were added to 2 mL water and the reaction mixture was stirred at 80 °C for the appropriate time, as mentioned in Table 8. After the completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, the solid product was obtained by simple filtration and the solid residue was finally recrystallized from EtOH.General procedure for the synthesis of 12a–d
A mixture of isatin 5 (1 mmol), malononitrile 3 (1.2 mmol), 2,3-dihydrophthalazine-1,4-dione 9 (1 mmol) and DCDBTSD (0.0455 g, 10 mol%) were added to 2 mL water and the reaction mixture was stirred at 80 °C for the appropriate time, as mentioned in Table 9. After the completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, the solid product was obtained by simple filtration and the solid residue was finally recrystallized from EtOH.Conclusions
In conclusion, we demonstrated that N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide (DCDBTSD), was a remarkably effective homogeneous catalyst for the one-pot construction of 4H-pyran, pyranopyrazole, pyrazolo[1,2-b] phthalazine and spiro-oxindoles derivatives in aqueous media from commercially available starting materials. The most noticeable feature within the study was that water was used both as a reaction medium and as a medium for the synthesis of the catalyst. Moreover, the aqueous conditions, outstanding yields, simple experimental procedure, environmentally friendly procedure and elimination of hazardous organic solvents are several advantages of this protocol.Spectral data analysis of compounds
Spiro[2-amino-4H-pyran-oxindole] (6a, Table 5)
White powder; mp 292–294 °C (lit: 303 °C). IR (KBr) (νmax, cm−1): 3361, 3297, 3197, 2206, 1734, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 712, 1675, 1360. 1H NMR (400 MHz, DMSO-d6) δppm: 6.869–6889 (d, 1H, J = 8 Hz, ArH), 6.938–6.976 (t, 1H, J = 7.6 Hz ArH), 7.225–7.241 (d, J = 6.4 Hz, 2H, ArH), 7.502–7.582 (m, 2H, ArH), 7.707 (s, 2H, NH2, D2O exchangeable), 7.768–7.806 (t, J = 7.2 Hz, 1H, ArH), 7.954–7.973 (d, J = 7.6 Hz, 1H, ArH), 10.717 (s, 1H, NH). 13C NMR (400 MHz, DMSO-d6): δppm 48.074, 57.494, 101.89, 109.973, 112.929, 117.138, 117.441, 122.524, 123.137, 124.600, 125.482, 129.390, 133.520, 134.140, 142.657, 152.51, 155.542, 158.741, 158.906, 177.615.
712, 1675, 1360. 1H NMR (400 MHz, DMSO-d6) δppm: 6.869–6889 (d, 1H, J = 8 Hz, ArH), 6.938–6.976 (t, 1H, J = 7.6 Hz ArH), 7.225–7.241 (d, J = 6.4 Hz, 2H, ArH), 7.502–7.582 (m, 2H, ArH), 7.707 (s, 2H, NH2, D2O exchangeable), 7.768–7.806 (t, J = 7.2 Hz, 1H, ArH), 7.954–7.973 (d, J = 7.6 Hz, 1H, ArH), 10.717 (s, 1H, NH). 13C NMR (400 MHz, DMSO-d6): δppm 48.074, 57.494, 101.89, 109.973, 112.929, 117.138, 117.441, 122.524, 123.137, 124.600, 125.482, 129.390, 133.520, 134.140, 142.657, 152.51, 155.542, 158.741, 158.906, 177.615.
Spiro[2-amino-4H-pyran-oxindole] (6b, Table 5)
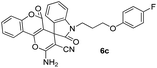
White powder; mp 278–280 °C (lit: 278–280 °C). IR (KBr) (νmax, cm−1): 3451, 3452, 3166, 2946, 2880, 2196, 1695, 1673, 1611, 1505, 1466, 1360, 762, 746. 1H NMR (400 MHz, DMSO-d6) δppm: 2.066–2.128 (m, 2H, CH2), 3.841–3.976 (m, 2H, CH2–N), 4.032–4.048 (d, 2H, J = 6.4 Hz, CH2–O), 6.941–6.971 (m, 2H, ArH), 6.989–7.026 (t, J = 7.6 Hz, 1H, ArH), 7.101–7.145 (m, 3H, ArH), 7.268–7.319 (m, 2H, ArH), 7.504–7.524 (d, J = 8 Hz, 1H, ArH), 7.553–7.591 (t, J = 7.6 Hz, 1H, ArH), 7.771–7.816 (m, 3H, NH2 and ArH, D2O exchangeable), 7.962–7.980 (d, J = 7.2 Hz, 1H, ArH). 13C NMR (400 MHz, DMSO-d6): δppm 27.27, 37.123, 47.616, 57.132, 65.708, 101.669, 108.970, 112.919, 116.127, 116.207, 116.287, 116.354, 117.184, 117.389, 123.186, 124.588, 125.539, 129.577, 132.739, 134.238, 143.346, 152.524, 155.246, 155.694, 155.753, 158.095, 158.825, 158.985, 176.109.Spiro[2-amino-4H-pyran-oxindole] (6c, Table 5)
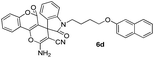
White powder; mp 270–272 °C. IR (KBr) (νmax, cm−1): 3482, 3452, 3166, 2946, 2880, 2196, 1695, 1673, 1611, 1505, 1466, 1360, 762, 746. 1H NMR (400 MHz, DMSO-d6) δppm: 2.066–2.113 (t, 2H, J = 6.4 Hz, CH2–O), 3.841–3.976 (m, 2H, CH2), 4.032–4.048 (d, 2H, J = 6.4 Hz, CH2–N), 6.926–6.960 (m, 2H, ArH), 6.989–7.026 (t, J = 7.6 Hz, 1H, ArH), 7.08–7.131 (m, 3H, ArH), 7.253–7.305 (m, 2H, ArH), 7.490–7.511 (d, J = 8.4 Hz, 1H, ArH), 7.540–7.577 (t, J = 7.6 Hz, 1H, ArH), 7.751–7.802 (m, 3H, NH2 and ArH), 7.944–7.966 (dd, J = 1.2 Hz, 1H, ArH). 13C NMR (400 MHz, DMSO-d6): δppm 27.27, 37.123, 47.616, 57.132, 65.708, 101.669, 108.970, 112.919, 116.127, 116.207, 116.287, 116.354, 117.184, 117.389, 123.186, 124.588, 125.539, 129.577, 132.739, 134.238, 143.346, 152.524, 155.246, 155.694, 155.753, 158.095, 158.825, 158.985, 176.109.Spiro[2-amino-4H-pyran-oxindole] (6d, Table 5)
Pale yellow powder; mp 268–270 °C. 1H NMR (400 MHz, DMSO-d6) δppm: 1.839–1.873 (br s, 4H, CH2), 3.794–3.824 (t, J = 6 Hz, 2H, CH2N), 4.127–4.155 (t, J = 5.6 Hz, 2H, CH2O), 7.002–7.039 (t, J = 7.6 Hz, 1H, ArH), 7.091–7.120 (dd, J = 2.4 Hz, 1H, ArH), 7.288–7.353 (m, 3H, ArH), 7.430–7.448 (d, J = 7.2 Hz, 2H, ArH), 7.467–7.496 (1H, ArH), 7.538–7.576 (t, J = 7.6 Hz, 1H, ArH), 7.637–7.657 (d, J = 8 Hz, 2H, ArH), 7.699 (br s, 2H, NH2, D2O exchangeable), 7.759–7.822 (m, 4H, ArH), 7.902–7.922 (d, J = 8 Hz, 1H, ArH), 7.958–7.977 (d, J = 7.6 Hz, 1H, ArH). 13C NMR (400 MHz, DMSO-d6): 23.948, 24.188, 26.387, 57.140, 67.391, 67.675, 101.726, 107.150, 117.165, 118.614, 119.225, 123.099, 123.184, 123.812, 123.909, 123.996, 126.120, 126.759, 126.830, 127.107, 127.953, 128.868, 129.571, 129.676, 132.855, 134.793, 146.983, 152.533, 155.664, 156.830, 156.994, 158.744, 176.089.Spiro[pyrano[2,3-c]pyrazole] (11a, Table 8)
White powder; mp 237–238 °C (lit: 236–237 °C). IR (KBr) (νmax, cm−1): 3410, 3287, 3124, 2202, 1692, 1655, 1526, 1132. 1H NMR (400 MHz, DMSO-d6) δppm: 1.560 (s, 3H, CH3),6.955–6.974 (d, J = 7.6 Hz, 1H, ArH), 7.03–7.067 (t, J = 7.6 Hz, 1H, ArH), 7.190–7.208 (d, J = 7.2 Hz, 1H, ArH), 7.286–7.324 (t, J = 7.6 Hz,2H, ArH), 7.353–7.390 (t, J = 7.2 Hz,1H, ArH), 7.516–7.556 (t, J = 8.4 Hz 2H, ArH), 7.612 (s, 2H, NH2, D2O exchangeable), 7.796–7.815 (d, J = 7.6 Hz 2H, ArH) 10.774 (s, 1H, NH). 13C NMR (400 MHz, DMSO-d6): δppm 12.17, 48.24, 56.62, 96.82, 110.32, 118.40, 118.43, 120.60, 123.12, 125.38, 127.05, 129.76, 129.94, 132.60, 137.71, 142.07, 144.42, 145.40, 161.46, 161.50, 177.98.Spiro[pyrano[2,3-c]pyrazole] (11b, Table 8)
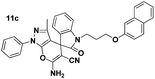
Pale yellow powder; mp 228–230 °C (lit: 232–234 °C). IR (KBr) (νmax, cm−1): 3390, 3314, 3191, 2904, 2200, 2208, 1701, 1662, 1396, 746. 1H NMR (400 MHz, DMSO-d6) δppm: 1.368 (s, 3H, CH3), 4.943–5.096 (AB-q, 2H, CH2), 7.094–7.130 (t, J = 7.6 Hz, 2H, ArH), 7.276–7.399 (m, 6H, ArH), 7.444–7.462 (d, J = 7.2 Hz, 2H, ArH), 7.523–7.563 (t, J = 7.6 Hz, 2H, ArH), 7.697 (s, 2H, NH2, D2O exchangeable), 7.801–7.821 (d, J = 8 Hz, 2H, ArH). 13C NMR (400 MHz, DMSO-d6): δppm 12.19, 43.79, 47.98, 56.33, 96.58, 110.00, 118.42, 120.67, 123.93, 125.33, 127.13, 128.08, 129.06, 129.83, 129.95, 131.82, 136.51, 137.67, 142.60, 144.33, 145.46, 161.59, 161.63, 161.67, 176.61.Spiro[pyrano[2,3-c]pyrazole] (11c, Table 8)
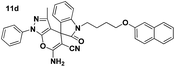
Yellow powder; mp 210–212 °C. IR (KBr) (νmax, cm−1): 3401, 3380, 3021, 2890, 2870, 2238, 1702, 1691, 1610, 1515, 1450, 1341, 1203, 755. 1H NMR (400 MHz, DMSO-d6) δppm: 1.516 (s, 3H, CH3), 2.152–2.303 (m, 2H, CH2), 3.933–4.06 (m, 2H, CH2N), 4.189–4.213 (t, J = 7.6 Hz, 2H, CH2O), 7.079–7.135 (t, J = 7.6 Hz, 1H, ArH), 7.168–7.304 (m, 4H, ArH), 7.344–7.393 (m, 3H, ArH), 7.444–7.480 (t, J = 7.2 Hz, 1H, ArH), 7.518–7.558 (t, J = 7.6 Hz, 1H, ArH), 7.697 (s, 2H, NH2, D2O exchangeable), 7.797–7.859 (m, 5H, ArH). 13C NMR (400 MHz, DMSO-d6): δppm 12.30, 26.94, 37.37, 37.63, 47.87, 56.47, 96.49, 107.23, 101.45, 118.65, 119.23, 120.65, 123.80, 124.06, 126.84, 127.11, 127.16, 127.99, 128.99, 129.76, 129.94, 132.02, 134.71, 137.68, 142.68, 144.34, 145.48, 156.78, 161.46, 161.46, 161.50, 176.33.Spiro[pyrano[2,3-c]pyrazole] (11d, Table 8)
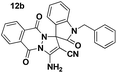
Yellow powder; mp 198–200 °C. IR (KBr) (νmax, cm−1): 3370, 3329, 3181, 2944, 2875, 2207, 1699, 1662, 1601, 1552, 1467, 1395, 1359, 1219, 751, 652. 1H NMR (400 MHz, DMSO-d6) δppm: 1.504 (s, 3H, CH3), 1901 (br s, 4H, CH2), 3.881–3.911 (t, J = 6 Hz 2H, CH2N), 4.151–4.179 (t, J = 6.4 Hz 2H, CH2O), 7.112–7.189 (m, 2H, ArH), 7.251–7.287 (t, J = 7.2 Hz, 2H, ArH), 7.329–7.406 (m, 4H, ArH), 7.439–7.476 (t, J = 7.6 Hz, 1H, ArH), 7.520–7.60 (t, d, J = 7.2 Hz, 2H, ArH), 7.647 (s, 2H, NH2), 7.798–7.837 (m, 5H, ArH); 13C NMR (400 MHz, DMSO-d6): δppm 12.28, 18.99, 19.04, 24.40, 26.55, 47.87, 56.39, 96.56, 107.16, 109.55, 118.23, 119.20, 120.64, 123.72, 123.97, 125.31, 126.82, 127.13, 127.96, 128.91, 129.73, 129.94, 131.96, 134.79, 137.68, 142.86, 144.31, 145.49, 156.98, 161.49, 161.54, 176.34.Spiro[indoline-3,1′-pyrazolo[1,2-b]phthalazine] (12b, Table 9)
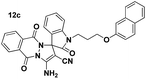
Yellow powder; mp 260–263 °C (lit: 265–266 °C). IR (KBr) (νmax, cm−1): 3310, 3270, 3020, 2895, 2229, 1720, 1666, 1662, 1593, 1494, 1349, 1083, 791, 759. 1H NMR (400 MHz, DMSO-d6) δH: 4.957 (s, 2H, CH2), 7.062–7.082 (d, J = 8 Hz, 1H, ArH), 7.191–7.231 (t, J = 8 Hz, 1H, ArH), 7.306–7.348 (m, 1H, ArH), 7.367–7.441 (m, 4H, ArH), 7.582–7.620 (t, J = 7.6 Hz, 1H, ArH), 7.892–7.925 (m, 3H, ArH), 7.962–7.981 (d, J = 7.6 Hz, 1H, ArH), 8.091 (br s, 3H, NH2 and ArH). 13C NMR (400 MHz, DMSO-d6): δppm 43.47, 82.12, 111.43, 111.99, 113.44, 118.72, 124.06, 125.61, 126.16, 127.88, 128.20, 129.19, 133.08, 135.70, 137.98, 146.57, 150.05, 163.12.Spiro[indoline-3,1′-pyrazolo[1,2-b]phthalazine] (12c, Table 9)
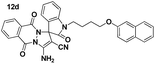
Yellow powder; mp 218–220 °C. IR (KBr) (νmax, cm−1): 3434, 3434, 3029, 2926, 2870, 2228, 1727, 1661, 1628, 1613, 1597, 1470, 1371, 1259, 1180, 837, 762, 748. 1H NMR (400 MHz, DMSO-d6) δppm: 2.136–2.184 (m, 2H, CH2), 3.917–3.951 (t, J = 6.8 Hz, 2H, CH2N), 4.164–4.193 (t, J = 5.6 Hz, 2H, CH2O), 7.094–7.122 (dd, J = 2.4 Hz, 1H, ArH), 7.170–7.208 (t, J = 7.6 Hz, 1H, ArH), 7.241–7.261 (m, 2H, ArH), 7.332–7.372 (m, 1H, ArH), 7.439–7.480 (m, 1H, ArH), 7.439–7.480 (m, 1H, ArH), 7.588–7.629 (m, 1H, ArH), 7.762–7.787 (d, J = 8 Hz 1H, ArH), 7.809–7.884 (m, 2H, ArH), 7.892–7.956 (m, 5H, ArH), 8.090 (br s, 2H, NH2); 13C NMR (400 MHz, DMSO-d6): δppm 26.93, 37.37, 37.63, 65.51, 81.50, 107.23, 111.08, 111.91, 113.43, 118.65, 119.06, 123.80, 124.06, 125.58, 126.10, 126.87, 127.11, 127.99, 128.96, 129.74, 133.10, 134.66, 138.11, 147.10, 150.30, 156.64, 163.00.Spiro[indoline-3,1′-pyrazolo[1,2-b]phthalazine] (12d, Table 9)
Yellow powder; mp 210–212 °C. IR (KBr) (νmax, cm−1): 3430, 3401, 3180, 2931, 2870, 2221, 1720, 1668, 1610, 1603, 1590, 1470, 1370, 1240, 1110, 768, 750, 718. 1H NMR (400 MHz, DMSO-d6) δppm: 1.846 (br s, 4H, 2CH2), 3.784–3.816 (t, J = 7.6 Hz, 2H, CH2N), 4.115–4.147 (t, J = 6.8 Hz, 2H, CH2O), 7.098–7.126 (dd, J = 2.4 Hz, 1H, ArH), 7.188–7.226 (t, J = 7.6 Hz, 1H, ArH), 7.249–7.268 (d, J = 8 Hz, 1H, ArH), 7.289–7.298 (d, J = 2 Hz, 1H, ArH), 7.327–7.363 (t, J = 7.2 Hz, 1H, ArH), 7.439–7.475 (t, J = 7.2 Hz, 1H, ArH), 7.645–7.683 (t, J = 7.6 Hz, 1H, ArH), 7.782–7.831 (m, 3H, ArH), 7.889–7.921 (m, 3H, ArH), 8.089 (br s, 2H, NH2); 13C NMR (400 MHz, DMSO-d6): δppm 23.97, 26.40, 37.35, 37.60, 67.39, 81.61, 107.15, 111.13, 111.94, 113.37, 118.59, 119.11, 123.82, 124.01, 125.58, 126.12, 126.84, 127.12, 127.97, 128.88, 129.73, 133.08, 134.73, 138.11, 146.96, 150.10, 156.80, 162.93.Acknowledgements
The authors acknowledge the Bu-Ali Sina university research council and Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for providing support to this study.Notes and references
- M. Khoobi, T. M. Delshad, M. Vosooghi, M. Alipour, H. Hamadi, E. Alipour, M. P. Hamedani, Z. Safaei, A. Foroumadi and A. Shafiee, J. Magn. Magn. Mater., 2015, 375, 217 CrossRef CAS PubMed.
- (a) A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, F. Abi, A. Zare, H. Kaveh, V. Khakyzadeh, M. Kazem-Rostami, A. Parhami and H. Torabi-Monfared, Tetrahedron, 2013, 69, 212 CrossRef CAS PubMed; (b) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare and V. Khakyzadeh, Appl. Catal., A, 2011, 400, 70 CrossRef CAS PubMed; (c) A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare, A. Zare, M. Khojasteh, Z. Asgari, V. Khakyzadeh and A. Khalafi-Nezhad, Catal. Commun., 2012, 20, 54 CrossRef CAS PubMed.
- L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517 CrossRef CAS.
- (a) A. R. Moosavi-Zare, M. A. Zolfigol, O. Khaledian, V. Khakyzadeh, M. D. Farahani and H. G. Krugerc, New J. Chem., 2014, 38, 2342 RSC; (b) T. S. Jin, A. Q. Wang, F. Shi, L. S. Han, L. B. Liu and T. S. Li, ARKIVOC, 2006, xiv, 78 Search PubMed; (c) R. Hekmatshoar, S. Majedi and K. Bakhtiari, Catal. Commun., 2008, 9, 307 CrossRef CAS PubMed; (d) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, J. Afsar, V. Khakyzadeh and O. Khalediana, J. Chin. Chem. Soc., 2015, 62, 398 CrossRef CAS PubMed; (e) M. Seifi and H. Sheibani, Catal. Lett., 2008, 126, 275 CrossRef CAS; (f) S. Balalaie, M. Bararjanian, A. M. Amani and B. Movassagh, Synlett, 2006, 263 CrossRef CAS.
- (a) A. Feurer, J. Luithle, S. Wirtz, G. Koenig, J. Stasch, E. Stahl, R. Schreiber, F. Wunder and D. Lang, PCT Int. Aool. Wo 2004009589, BayeHealtheare Ag, Germany; (b) M. N. Nasr and M. M. Gineinah, Arch. Pharm., 2002, 335(6), 289–295 CrossRef CAS; (c) V. K. Ahluwalia, A. Dahiya and V. Garg, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1997, 36, 88 Search PubMed; (d) M. R. Nadia, Y. K. Nahed, A. Fahmyb and A. A. F. El-Sayeda, Der Pharma Chemica, 2010, 2, 400 Search PubMed.
- A. Siddekha, A. Nizam and M. A. Pasha, Spectrochim. Acta, Part A, 2011, 81, 431 CrossRef CAS PubMed.
- (a) A. R. Moosavi-Zare, M. A. Zolfigol, E. Noroozizadeh, M. Tavasoli, V. Khakyzadeh and A. Zare, New J. Chem., 2013, 37, 4089 RSC; (b) H. V. Chavan, S. B. Babar, R. U. Hoval and B. P. Bandgar, Bull. Korean Chem. Soc., 2011, 32, 3963 CrossRef CAS; (c) N. M. H. Elnagdi and N. S. Al-Hokbany, Molecules, 2012, 17, 4300 CrossRef CAS PubMed; (d) M. A. Zolfigol, M. Tavasoli, A. R. Moosavi-Zare, P. Moosavi, H. G. Kruger, M. Shiri and V. Khakyzadeh, RSC Adv., 2013, 3, 25681 RSC.
- M. Asif, Curr. Med. Chem., 2012, 19, 2984 CrossRef CAS.
- (a) M. Kidwai, A. Jahan, R. Chauhan, N. Mishra and K. Neeraj, Tetrahedron Lett., 2012, 53, 1728 CrossRef CAS PubMed; (b) E. Mosaddegh and A. Hassankhani, Tetrahedron Lett., 2011, 52, 488 CrossRef CAS PubMed; (c) R. Fazaeli, H. Aliyan and N. Fazaeli, Open Catal. J., 2010, 3, 14 CrossRef CAS; (d) H. R. Shaterian, F. Khorami, A. Amirzadeh, R. Doostmohammadi and M. Ghashang, Journal of the Iranian Chemical Research, 2009, 2, 57 Search PubMed; (e) H. Hamidian, S. Fozooni, A. Hassankhani and S. Z. Mohammadi, Molecules, 2011, 16, 9041 CrossRef CAS PubMed; (f) M. Sayyafi, M. Seyyedhamzeh, H. R. Khavasi and A. Bazgir, Tetrahedron, 2008, 64, 2375 CrossRef CAS PubMed.
- E. Kolvari, A. Ghorbani-Choghamarani, P. Salehi, F. Shirini and M. A. Zolfigol, J. Iran. Chem. Soc., 2007, 4, 126 CrossRef CAS.
- H. Veisi and R. Ghorbani-Vaghei, Tetrahedron, 2010, 66, 7445 CrossRef CAS PubMed.
- A. Khazaei, F. Abbasi and A. R. Moosavi-Zare, New J. Chem., 2014, 38, 5287 RSC.
- (a) A. E. Medvedev, A. Clow, M. Sandler and V. Glover, Biochem. Pharmacol., 1996, 52, 385 CrossRef CAS; (b) A. Khazaei, A. Alizadeh and R. G. Vaghei, Molecules, 2001, 6, 253 CrossRef CAS PubMed.
- A. Khazaei, A. Raiatzadeh and A. Rostami, J. Chin. Chem. Soc., 2007, 54, 465 CrossRef CAS PubMed.
- A. Khazaei, F. Abbasi and A. R. Moosavi-Zare, RSC Adv., 2014, 4, 1388 RSC.
- A. Khazaei, F. Abbasi, M. K. borazjani and S. Saednia, J. Braz. Chem. Soc., 2014, 25, 361 CAS.
- A. Khazaei and R. Ghorbani-Vaghei, Asian J. Chem., 2000, 12, 584 CAS.
- A. Khazaei, R. G. Vaghei and M. A. Zolfigol, Asian J. Chem., 2003, 15, 591 CAS.
- A. Khazaei, A. Rostami, Z. Tanbakouchian and Z. Zinati, Catal. Commun., 2006, 7, 214 CrossRef CAS PubMed.
- A. Khazaei, A. A. Manesh and A. H. Ghasemi, Synthesis, 2004, 2784 CrossRef CAS.
- A. Khazaei, M. Sadri and E. Mehdipour, Iran. Polym. J., 1996, 5, 1026 Search PubMed.
- S. Abdolmohammadi and S. Balalaie, Tetrahedron Lett., 2007, 48, 3299 CrossRef CAS PubMed.
- H. J. Wang, J. Lu and Z.-H. Zhang, Monatsh. Chem. Chem. Mon., 2010, 141, 1107 CrossRef CAS.
- Y. Wang, J. Luo, T. Xing and Z. Liu, Monatsh. Chem. Chem. Mon., 2013, 144, 1871 CrossRef CAS.
- X.-Y. Meng, H.-J. Wang, C.-P. Wang and Z.-H. Zhang, Synth. Commun., 2011, 41, 3477 CrossRef CAS PubMed.
- F. Kargar Behbahani, M. Ghorbani, M. Sadeghpour and M. Mirzaei, Lett. Org. Chem., 2013, 10, 191 CrossRef.
- M. G. Dekamin and M. Eslami, Green Chem., 2014, 16, 4914 RSC.
- J. Wang, D. Shi, Q. Zhuang, X. Wang and H. Hu, J. Chem. Res., 2004, 2004, 818 CrossRef.
- S. Khodabakhshi, B. Karami, K. Eskandari and S. J. Hoseini, C. R. Chim., 2014, 17, 35 CrossRef CAS PubMed.
- Y. El-Ossaily, R. Zaki and S. Metwally, J. Sci. Res., 2014, 6, 293 Search PubMed.
- R. M. Williams and R. J. Cox, Acc. Chem. Res., 2003, 36, 127 CrossRef CAS PubMed.
- J. F. Da Silva, S. J. Garden and A. C. Pinto, J. Braz. Chem. Soc., 2001, 12, 273 CrossRef CAS.
- M.-Y. Chang, C.-L. Pai and Y.-H. Kung, Tetrahedron Lett., 2005, 46, 8463 CrossRef CAS PubMed.
- P. S. Baran and J. M. Richter, J. Am. Chem. Soc., 2005, 127, 15394 CrossRef CAS PubMed.
- S. T. Hilton, T. C. Ho, G. Pljevaljcic and K. Jones, Org. Lett., 2000, 2, 2639 CrossRef CAS.
- T. Kosuge, K. Tsuji, K. Hirai, K. Yamaguchi, T. Okamoto and Y. Iitaka, Tetrahedron Lett., 1981, 22, 3417 CrossRef CAS.
- M. J. Kornet and A. P. Thio, J. Med. Chem., 1976, 19, 892 CrossRef CAS.
- J. Feng, K. Ablajan and A. Sali, Tetrahedron, 2014, 70, 484 CrossRef CAS PubMed.
- C. M. Clay, H. M. Abdallah, C. Jordan, K. Knisley and D. M. Ketcha, ARKIVOC, 2012, 6, 317 Search PubMed.
- (a) A. R. Karimi and F. Sedaghatpour, Synthesis, 2010, 1731 CrossRef CAS; (b) A. R. Karimi, M. Sourinia, Z. Dalirnasab and M. Karimi, Can. J. Chem., 2015 Search PubMed.
- (a) A. Rahmati and Z. Khalesi, Tetrahedron, 2012, 68, 8472 CrossRef CAS PubMed; (b) M. Babaie and H. Sheibani, Arabian J. Chem., 2011, 4, 159 CrossRef CAS PubMed.
- Z. K. Jaberi, M. M. R. Shams and B. Pooladian, Acta Chim. Slov., 2013, 60, 105 Search PubMed.
- K. Eskandari, B. Karami and S. Khodabakhshi, Catal. Commun., 2014, 54, 124 CrossRef CAS PubMed.
- A. P. G. Nikalje and S. I. Shaikh, Ultrasound-promoted synthesis of indole appended heterocycles world journal of pharmacy and pharmaceutical sciences, World J. Pharm. Pharm. Sci., 2014, 3(4), 1282–1302 CAS , ISSN 2278–4357.
- K. Ghodrati, S. H. Hosseini, R. Mosaedi, C. Karami, F. Maleki, A. Farrokhi and Z. Hamidi, Int. Nano Lett., 2013, 3, 1 CrossRef.
- R. S. Joshi, P. G. Mandhane, S. D. Diwakar and C. H. Gill, Ultrason. Sonochem., 2010, 17, 298 CrossRef CAS PubMed.
- J.-T. Li, M.-X. Sun, G.-Y. He and X.-Y. Xu, Ultrason. Sonochem., 2011, 18, 412 CrossRef CAS PubMed.
- J.-T. Li, H.-G. Dai, W.-Z. Xu and T.-S. Li, Ultrason. Sonochem., 2006, 13, 24 CrossRef CAS PubMed.
- M. M. Heravi, S. Sadjadi, S. Sadjadi, H. A. Oskooie and F. F. Bamoharram, Ultrason. Sonochem., 2009, 16, 718 CrossRef CAS PubMed.
- Y. Mo, H. Zang and B. Cheng, 2010 International Conference on Mechanic Automation and Control Engineering, 2010 Search PubMed.
- H. Kefayati, S. H. Amlashi, R. Kazemi-Rad and A. Delafrooz, C. R. Chim., 2014, 17, 894 CrossRef CAS PubMed.
- M. Kidwai and R. Chauhan, J. Heterocycl. Chem., 2014, 51, 1689 CrossRef CAS PubMed.
- H. R. Shaterian and M. Mohammadnia, Res. Chem. Intermed., 2014, 40, 371 CrossRef CAS.
- J. Safaei-Ghomi, H. Shahbazi-Alavi, A. Ziarati, R. Teymuri and M. R. Saberi, Chin. Chem. Lett., 2014, 25, 401 CrossRef CAS PubMed.
- A. Dandia, V. Parewa, A. K. Jain and K. S. Rathore, Green Chem., 2011, 13, 2135 RSC.
- A. Dandia, D. Saini, S. Bhaskaran and D. K. Saini, Med. Chem. Res., 2014, 23, 725 CrossRef CAS.
- X.-N. Zhang, Y.-X. Li and Z.-H. Zhang, Tetrahedron, 2011, 67, 7426 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra10730j |
| This journal is © The Royal Society of Chemistry 2015 |