PbSe nanocrystal solar cells using bandgap engineering
Xiaoyu Zhangac,
Yu Zhang*ab,
Long Yana,
Hua Wua,
Wenzhu Gaob,
Jun Zhaocd and
William W. Yu*acd
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, China. E-mail: yuzhang@jlu.edu.cn; wyu6000@gmail.com
bState Key Laboratory of Superhard Materials, College of Physics, Jilin University, Changchun 130012, China
cCollege of Material Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, China
dDepartment of Chemistry and Physics, Louisiana State University, Shreveport, LA 71115, USA
First published on 24th July 2015
Abstract
Benefiting from the strong quantum confinement, PbSe nanocrystals allow their bandgap and absorption edge to be tuned to optimize the absorption of the solar radiation. Here, bandgap engineering-based photovoltaic devices were designed, fabricated, and characterized using two-size PbSe nanocrystals. The fabricated two size particle photovoltaic devices showed 12.8% higher power conversion efficiency compared to that of the single-particle devices, as a result of the enhanced photon absorption and the improved charge transfer.
1. Introduction
Semiconductor nanocrystals (NCs) have generated great interest worldwide for use in photovoltaic devices,1–7 due to their facile solution processing, low cost, and quantum-size-effect bandgap tunability. Ever since the first NC solar cells were demonstrated,8 the device power conversion efficiency has been steadily improved to greater than 8.5%,9–11 as a result of the efforts based on the advanced engineering in materials and device architectures.12–14Among the variety of NCs, PbSe NCs have shown their unique properties. They possess high absorption coefficients and can be easily tuned to absorb light from 0.6 to 4 μm with size varied from 1.1 to 17 nm.6,15–19 PbSe-based NC solids have shown high carrier mobility of 10 cm2 V−1 s−1,20 and a fairly low density of intragap defects near commonly used metal-oxide window layers.21 Furthermore, the PbSe NCs feature enhanced multiple exciton generation (MEG) or carrier multiplication (CM),22–25 and the MEG-enhanced photocurrent has been demonstrated in PbSe NC solar cells only.26 Recently, air stable PbSe NC solar cells have gained power conversion efficiency (PCE) exceeding 6%.27
Up to now, photovoltaics based on PbSe NCs have been focused on single-junction solar cells. Multi-junction solar cells made from a combination of NCs in different sizes will increase the energy harvesting from the broad solar radiation.28 However, the processing procedure of a tandem solar cell is complicated, and an efficient recombination layer in connection of the front and back cells for hole and electron current recombining is essential, which is not easy to fabricate.29,30 In addition, current matching is also required in order to optimize the tandem cell, which is in need of theoretical calculations and a large amount of experimental practices.28
In order to take better use of the solar energy, photovoltaic device using bandgap engineering is a good alternative, not only for broadening absorption as tandem cells do, but also for the easier production procedure needed than tandem devices. Bandgap engineering has been employed in producing charge transport/blocking layers in organic light-emitting diodes or organic solar cells, and is effective in improving photovoltaic performance.31–35 Besides, the using of a quantum funnel to improve the photoelectron transportation in colloidal quantum dot based solar cells has also been reported.36
In this paper, simple device structure was designed using band alignment engineering. The bandgap engineered solar cells could extend the absorption spectrum of the active layer and contribute an easier device production process than tandem cells. Compared to the single-particle control device, the enhanced photon absorption leaded to a higher short-circuit current for the two-size-particle device. Together with the improved fill factor (FF) which is benefited from band engineering, the solar cell performance was obviously improved.
2. Experimental section
2.1 Chemicals
Lead(II) oxide (PbO; 99.99%) and sodium hydroxide (98%) were purchased from Alfa Aesar. Oleic acid (OA; 90%), 1-octadecene (ODE; 90%), tributylphosphine (TBP; 97%), selenium (Se; 100 mesh, 99.99%), zinc acetate (99.99%), methanol, octane, hexane, butyl alcohol, acetonitrile and ethyl alcohol were obtained from Aldrich. 1,2-Ethanedithiol (EDT, >98%) was acquired from Fluka.2.2 Synthesis of PbSe NCs
PbSe NCs were synthesized by modifying the previously reported methods.37,38 All manipulations were carried out in a water and oxygen free environment. PbO (0.892 g), OA (5 mL) and ODE (12 mL) were loaded into a three necked flask equipped with condenser, magnetic stirrer, thermocouple, and heating mantle. After 10 min nitrogen flow to replace the air, the flask was heated to 180 °C. The solution became colorless in an hour. For the synthesis of 2.6 nm (2.9 nm) nanoparticles, 8 mL 1.0 M TBP-Se was injected into the reaction flask swiftly at 90 °C under nitrogen atmosphere. The reaction temperature quickly dropped and was kept at 75 °C. The color of the mixture changed from nearly colorless to dark brown, indicating the formation of PbSe NCs. 50 mL methanol was injected into the reaction flask to quench the reaction after 3 min (3.5 min), and the original aliquots were preliminary purified by centrifugation. Then, a mass of methanol was added and the mixture was centrifuged for 10 minutes at 5000 rpm. 2 mL octane was put into the suspension to dissolve the NCs, abundant butyl alcohol was loaded in and then the NCs were isolated by centrifugation.39,40 In the end, the NCs were dispersed in octane and stored in a refrigerator.2.3 Synthesis of ZnO NCs
The ZnO NCs were synthesized using our previously reported procedure.41,42 A mixture of 0.4403 g zinc acetate and 30 mL ethyl alcohol were loaded into a 250 mL three-neck flask. After 10 min nitrogen flow, the three-neck flask was heated with stirring to make the mixture boiling. After 30 min, zinc acetate powder completely disappeared and the solution became colorless and transparent. Then, the three-neck flask was cooled to room temperature naturally and the solution turned into a white turbid solution. 0.2 g of sodium hydroxide in 10 mL ethyl alcohol was injected into the flask swiftly and the stirring was continued for another 4 h. A series of purification operation procedures were then carried out. Typically, 5 mL ZnO NC solution was loaded into a 50 mL centrifuge tube and hexane was added to full. After centrifugation the precipitate was dissolved in 3 mL ethyl alcohol. The purification was executed one more time and the finally obtained ZnO NCs were dissolved in 3 mL ethyl alcohol and stored in a nitrogen filled glove box.2.4 Device fabrication
Patterned ITO coated glass was cleaned with soap, deionized water, ethanol, chloroform, acetone, and isopropanol successively, and then was treated in UV-ozone for 15 min. A layer of poly(ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS; ∼40 nm thick) was spin-coated on ITO and annealed for 10 min at 150 °C in air. The 1,2-ethanedithiol (EDT) treated PbSe NC film was spin-coated on PEDOT:PSS layer in a N2 glove box through a layer-by-layer procedure. Normally, we first spin-cast the PbSe NC octane solution (12 mg mL−1) at 1000 rpm for 40 seconds, then soaked the deposited film with 0.1 M EDT solution in anhydrous acetonitrile for 1 min and then spin. Wash the EDT treated PbSe NC film with pure acetonitrile, and octane successively. Then repeat the NC spin procedure until desired thickness was reached. ZnO NC film was deposited over PbSe active region by spin coating, and was annealed at 90 °C for 30 min in the glove box. The device was completed by vacuum deposition of an Al cathode (100 nm thick) in a high-vacuum chamber (background pressure ∼1 × 10−6 Torr). The active area of the device was 4 mm2 as defined by the patterned electrodes.2.5 Characterizations
Transmission electron microscopy (TEM) images of the purified PbSe and ZnO NCs were obtained on a FEI Tecnai F20 microscope. Absorption spectra were measured on a Perkin-Elmer Lambda 950 UV-vis-NIR spectrometer and photoluminescence (PL) spectra on a Cary Eclipse spectrofluorimeter.Current–voltage characteristics of the solar cells in the dark and under simulated 1-sun illumination were measured using a Keithley 2612B source meter. 50 points were collected per 100 mV, with a scanning speed of 200 mV s−1. The scanning direction was from −0.5 V to +0.5 V. The light intensity from the solar simulator was determined using a calibrated single-crystalline silicon reference cell. A Crowntech QTest Station 1000AD was used to measure the external quantum efficiency.
3. Results and discussion
Fig. 1 shows the schematics of the device structures employed in this work. It has been proven that solar cells employed PbSe NCs with a first excitonic absorption peak at a wavelength λ = 870 nm (first exciton energy of 1.42 eV) could be well performed,3,13 thus the oleic acid capped PbSe NCs with first exciton energy of 1.42 eV (1.35 eV) in solution were used to fabricate the control device, named Cell 1 (Cell 2). The active layer of Cell 1 had a thickness of 70 nm, and the bulky ligands of the PbSe NCs were replaced with short ligands through the EDT treatment. All process occurred in a nitrogen glove box. For the devices using band engineering (two sized particles, Cell BE), the active layers consist two compositions. At first, a 30 nm thick film of 1.42 eV PbSe NCs was deposited upon the PEDOT:PSS layer; then the rest part of the active layer (40 nm) was prepared using NCs with the first exciton energy of 1.35 eV. Except for the active layer of Cell 1 and Cell BE, the other parts of the two devices were all the same. UV-vis absorption spectra of the employed NCs are shown in Fig. 2, together with their transmission electron microscope (TEM) images. The TEM micrographs of the PbSe NCs show that these NCs were single crystals without detectable stacking faults or crystal defects.The J–V characteristics of photovoltaic devices are shown in Fig. 3a. Cell 1 shows a power conversion efficiency of 3.20 ± 0.3%, and a higher open circuit voltage (VOC) compared to Cell BE. Meanwhile, Cell BE gives a higher short-circuit current density (JSC) and a larger fill factor (FF), resulting in a 12.8% improvement in power conversion efficiency to 3.61 ± 0.3%. In order to find out the origin of the increase in JSC, the external quantum efficiency (EQE) of the devices was measured, as given in Fig. 3b. As shown, a clear contribution originated from 1.35 eV PbSe NCs can be seen, confirming that Cell BE has extended the absorption spectrum and hence enhanced the short-circuit current.
 | ||
| Fig. 3 (a) Current density–voltage characteristics of Cell 1, Cell 2 and Cell BE, with their EQE curves shown in (b). | ||
The improvement in FF can be attributed to the band offsets between the two sized PbSe NC layers. Fig. 4a shows the energy level diagram of the component materials used in the devices. The conductive polymer PEDOT:PSS has a highest occupied molecular orbital (HOMO) energy of −5.0 eV, and functions as a hole transport layer (HTL) for facilitating the collection of holes from NCs. Meanwhile, it also blocks the electron transport at NC/HTL interface, which is benefited from its lowest unoccupied molecular orbital (LUMO) energy of −3.4 eV. The layer-by-layer processed active layer has a thickness of 70 nm, which is well below the effective exciton diffusion length of about 100 nm.43 The electron donor film with such thickness will generate fewer traps than thick films, which is beyond the exciton diffusion length, thus efficient carrier transportation can be ensured. ZnO is a commonly used electron acceptor, with a fairly low density of intragap defects when constructing a bilayer solar cell with PbSe NCs.21 With the help of its low HOMO energy of −7.4 eV, the ZnO film here not only functions as an electron transport layer (ETL), but also serves as a hole blocking layer.
 | ||
| Fig. 4 (a) Flat-band energy level diagram, with PbSe NC energy levels shown as an inset. Energy level values were taken from ref. 41. Schematic illustration of proposed band bending in Cell 1 (b) and Cell BE (c) at short-circuit conditions. | ||
In previously studies, injection of photo-excited electrons from colloidal NCs to electron acceptors has been investigated.44,45 From the Marcus theory, the increased offset in heterojunction energy level alignment would provide increased charge transfer.46–48 It has been proven that for PbSe NCs with higher LUMO compared to that of ZnO NCs, the photo-excited electrons can be injected into ZnO NCs efficiently.21,49 As the NC energy levels shown in Fig. 4a, band offset between the LUMO of PbSe NCs and the LUMO of ZnO NCs thus can provide a driving force for the flow of electrons. In both Cell 1 and Cell BE, their electron transportation benefit from this driving force.
As shown in Fig. 3a, Cell BE has a higher FF than that of Cell 1. The reason for this improvement is due to the interaction between the energy levels of the two NCs. As the hole effective-mass is greater than electron effective-mass, the electron affinity varies much more rapidly than ionization potential when the PbSe NC size changes.44,49 For the 1.42 eV and 1.35 eV NCs, larger difference between their LUMO energy than the HOMO energy could be found, as show in the insert of Fig. 4a. Thus the grading will appear primarily in the conduction band when the two PbSe layers are putting together. Schematic illustration of proposed band bending in Cell 1 and Cell BE at short-circuit conditions are given in Fig. 4b and c, respectively. As shown in the bandgap engineered device, the conduction band offset between the two PbSe NC layers provides an additional driving force for electrons, whereas the holes transportation keeps efficient in the relatively flat valence bands. Meanwhile, the higher LUMO of the 1.42 eV PbSe NCs also helps to prevent photo-generated electrons within the 1.35 eV PbSe layer from flowing to the anode, so that carrier recombination at the PbSe/anode interface can be reduced, which will lead to improved photocurrent collection efficiency. All together, the replacement of the upper 40 nm of the active layer with larger PbSe NCs not only extended the device absorption width but also improved the charge carrier transportation and enhanced the photocurrent collection efficiency. With the help of all these advantages, solar cell performance was obviously been improved.
In order to have a better understanding of the charge separation and transfer dynamics between the two PbSe NCs, the PL spectroscopy was developed. As shown in Fig. 5, the PL spectra of the 1.42 eV and 1.35 eV PbSe NCs peaked at a wavelength around 967 nm and 1042 nm, respectively. Then a mixture of the two PbSe NCs with a blending ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt/wt) was prepared. The PL curve of the mixture exhibits similar shape with that of the large-size NCs. All these indicate that both the photoelectrons and holes can be successfully funneled into the small-gap NCs. Besides, the 1.35 eV PbSe layer tends to play a dominant role in determination of the open-circuit voltage in Cell BE, so that the decrease in VOC has been observed. Taken together of the interfacial band bending of the active layer, Cell BE thus offers a median VOC value compared to that of devices employed 1.35 eV or 1.42 eV NCs, respectively.
1 (wt/wt) was prepared. The PL curve of the mixture exhibits similar shape with that of the large-size NCs. All these indicate that both the photoelectrons and holes can be successfully funneled into the small-gap NCs. Besides, the 1.35 eV PbSe layer tends to play a dominant role in determination of the open-circuit voltage in Cell BE, so that the decrease in VOC has been observed. Taken together of the interfacial band bending of the active layer, Cell BE thus offers a median VOC value compared to that of devices employed 1.35 eV or 1.42 eV NCs, respectively.
To gain more insight into this designed device structure, devices without ZnO films were fabricated. As the J–V characteristic curves in Fig. 6a shown, an obvious decrease in solar cell performance can be found in devices without ZnO layers. The absence of ZnO film leads to a serious leakage current at the negative bias, and made the device suffering from a poor rectification factor.
 | ||
| Fig. 6 (a) Current density–voltage characteristics of devices with or without ZnO films. (b) Total absorption of devices with or without ZnO layer measured in a reflection geometry. | ||
For the efficiency loss of the devices without ZnO, two factors are summarized, including the unbalanced charge-carrier transport and reduced photon absorption. The ZnO film here not only served for blocking holes and transporting electrons, but also improved the light harvesting. As shown in Fig. 6b, the insertion of the transparent ZnO layer between active layer and aluminum electrode had obviously enhanced the photon absorption by changing the optical interference between the incident light and the reflected light from aluminum electrode.50,51 This redistribution of the light intensity within the active layer caused the improved light harvesting.
Previously reported study have shown that fresh PbSe NC based solar cells will become ineffective within 3 minutes,43 indicating that devices employed large NCs are unstable under air condition. Nevertheless, as shown in Fig. 7, cells fabricated using small NCs largely improve the stability. This is originated from the significant difference in the composition of NC surfaces.52 Within the first 6 hours, the device PCE kept on increasing, and reduced to half after 48 hours. The open-circuit voltage value was enlarged during the whole process, while the short-circuit current and fill factor started going small 6 hours later. It has been proven that the first absorption peak of EDT-treated PbSe film progressively blue-shifts with time under air condition,53,54 which implies that the optical bandgap is becoming larger (higher LUMO and lower HOMO). Thus the difference between HOMO of PbSe and LUMO of ZnO is becoming larger and larger, and contributes to a higher VOC. Meanwhile, the higher LUMO of PbSe will make electron injection to electron acceptors better, and this brings about an improved FF. The higher JSC is benefited from the improved charge mobility, as EDT-treated PbSe film will become 1500–5000 times more conductive after air exposure.53 The decline of device efficiency starts from the sixth hours, which reveals the destruction of the active layer and interfaces from the oxygen attack. In short, enhanced air stability was obtained, showing a great potential for solar cells employing small PbSe NCs.
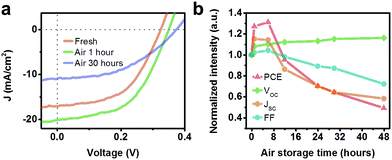 | ||
| Fig. 7 (a) J–V curves of Cell BE for different storage times in air, and (b) the normalized performance parameters. | ||
Averaged photovoltaic parameters out of more than 10 devices are summarized in Table 1. In addition, the Cell 2 represents for devices whose active layers were fabricated from 1.35 eV PbSe NCs. As expected, Cell 2 gives the highest JSC and the lowest VOC among these devices. The JSC benefits from the best absorption of Cell 2, while the VOC is related to the highest HOMO level of the active layer of Cell 2. The best 1-sun power conversion efficiency of 3.91% was obtained from Cell BE, indicating that the designed device structure really helped in improving photovoltaic efficiencies.
| VOC (V) | JSC (mA cm−2) | FF | PCE (%) | |
|---|---|---|---|---|
| Cell 1 | 0.41 ± 0.2 | 17.3 ± 3.1 | 46 ± 4 | 3.20 ± 0.3 |
| Cell 2 | 0.32 ± 0.2 | 22.4 ± 3.9 | 44 ± 3 | 3.09 ± 0.3 |
| Cell BE | 0.36 ± 0.3 | 20.1 ± 3.3 | 50 ± 3 | 3.61 ± 0.3 |
| Cell BE (best) | 0.35 | 21.5 | 52 | 3.91 |
4. Conclusions
In summary, well performed PbSe NC solar cells using bandgap engineering were fabricated successfully. Devices employed this designed structure could simultaneously extend the range of light-harvesting, improve the charge carrier transportation, and enhance the photocurrent collection efficiency, resulting in a 12.8% improvement in power conversion efficiency. Importantly, this bandgap engineered photovoltaics can broaden the device absorption spectrum, which is similar to the multi-junction photovoltaic devices, but no need to suffer from the complex device fabrication process. The best performing solar cell gave a PCE of 3.91%, with an improved FF value of 52%. All these positive results show that the designed device structure is effective, and contribute a briefly thinking for designing photovoltaic device structures.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (61106039, 51272084, 61306078, 61225018, 61475062), the Jilin Province Key Fund (20140204079GX), the State Key Laboratory on Integrated Optoelectronics (IOSKL2012ZZ12) and State Key Laboratory on Integrated Optoelectronics Fund open topics (IOSKL2014KF18), the Taishan Scholarship, the Shandong Natural Science Foundation (ZR2012FZ007), NSF (1338346), and NSF (2015)-LINK.Notes and references
- J. H. Bang and P. V. Kamat, ACS Nano, 2009, 3, 1467–1476 CrossRef CAS PubMed.
- Z. Chen, H. Zhang, Q. Zeng, Y. Wang, D. Xu, L. Wang, H. Wang and B. Yang, Adv. Energy Mater., 2014, 4, 1400235 Search PubMed.
- R. L. Hoye, B. Ehrler, M. L. Böhm, D. Muñoz-Rojas, R. M. Altamimi, A. Y. Alyamani, Y. Vaynzof, A. Sadhanala, G. Ercolano and N. C. Greenham, Adv. Energy Mater., 2014, 4, 1301544 Search PubMed.
- J. Jean, S. Chang, P. R. Brown, J. J. Cheng, P. H. Rekemeyer, M. G. Bawendi, S. Gradečak and V. Bulović, Adv. Mater., 2013, 25, 2790–2796 CrossRef CAS PubMed.
- S. Kim, M. Kang, S. Kim, J.-H. Heo, J. H. Noh, S. H. Im, S. I. Seok and S.-W. Kim, ACS Nano, 2013, 7, 4756–4763 CrossRef CAS PubMed.
- W. Ma, S. L. Swisher, T. Ewers, J. Engel, V. E. Ferry, H. A. Atwater and A. P. Alivisatos, ACS Nano, 2011, 5, 8140–8147 CrossRef CAS PubMed.
- S. M. Willis, C. Cheng, H. E. Assender and A. A. Watt, Nano Lett., 2012, 12, 1522–1526 CrossRef CAS PubMed.
- S. A. McDonald, G. Konstantatos, S. Zhang, P. W. Cyr, E. J. D. Klem, L. Levina and E. H. Sargent, Nat. Mater., 2005, 4, 138–142 CrossRef CAS PubMed.
- C.-H. M. Chuang, P. R. Brown, V. Bulović and M. G. Bawendi, Nat. Mater., 2014, 13, 796–801 CrossRef CAS PubMed.
- M. Yuan, O. Voznyy, D. Zhitomirsky, P. Kanjanaboos and E. H. Sargent, Adv. Mater., 2015, 27, 917–921 CrossRef CAS PubMed.
- D. Zhitomirsky, O. Voznyy, L. Levina, S. Hoogland, K. W. Kemp, A. H. Ip, S. M. Thon and E. H. Sargent, Nat. Commun., 2014, 5, 3803 CAS.
- Z. Ning, O. Voznyy, J. Pan, S. Hoogland, V. Adinolfi, J. Xu, M. Li, A. R. Kirmani, J.-P. Sun and J. Minor, Nat. Mater., 2014, 13, 822–828 CrossRef CAS PubMed.
- D. K. Ko, P. R. Brown, M. G. Bawendi and V. Bulović, Adv. Mater., 2014, 26, 4845–4850 CrossRef CAS PubMed.
- J. Zhang, J. Gao, E. M. Miller, J. M. Luther and M. C. Beard, ACS Nano, 2013, 8, 614–622 CrossRef PubMed.
- Q. Dai, Y. Wang, X. Li, Y. Zhang, D. J. Pellegrino, M. Zhao, B. Zou, J. Seo, Y. Wang and W. W. Yu, ACS Nano, 2009, 3, 1518–1524 CrossRef CAS PubMed.
- F. W. Wise, Acc. Chem. Res., 2000, 33, 773–780 CrossRef CAS PubMed.
- W. Hu, R. Henderson, Y. Zhang, G. You, L. Wei, Y. Bai, J. Wang and J. Xu, Nanotechnology, 2012, 23, 375202 CrossRef PubMed.
- Y. Zhang, Q. Dai, X. Li, B. Zou, Y. Wang and W. W. Yu, J. Nanopart. Res., 2011, 13, 3721–3729 CrossRef CAS.
- J. M. Pietryga, R. D. Schaller, D. Werder, M. H. Stewart, V. I. Klimov and J. A. Hollingsworth, J. Am. Chem. Soc., 2004, 126, 11752–11753 CrossRef CAS PubMed.
- S. J. Oh, N. E. Berry, J.-H. Choi, E. A. Gaulding, T. Paik, S.-H. Hong, C. B. Murray and C. R. Kagan, ACS Nano, 2013, 7, 2413–2421 CrossRef CAS PubMed.
- B. Ehrler, K. P. Musselman, M. L. Böhm, F. S. Morgenstern, Y. Vaynzof, B. J. Walker, J. L. MacManus-Driscoll and N. C. Greenham, ACS Nano, 2013, 7, 4210–4220 CrossRef CAS PubMed.
- M. C. Beard, J. M. Luther, O. E. Semonin and A. J. Nozik, Acc. Chem. Res., 2012, 46, 1252–1260 CrossRef PubMed.
- I. Gdor, H. Sachs, A. Roitblat, D. B. Strasfeld, M. G. Bawendi and S. Ruhman, ACS Nano, 2012, 6, 3269–3277 CrossRef CAS PubMed.
- L. A. Padilha, J. T. Stewart, R. L. Sandberg, W. K. Bae, W.-K. Koh, J. M. Pietryga and V. I. Klimov, Acc. Chem. Res., 2013, 46, 1261–1269 CrossRef CAS PubMed.
- R. D. Schaller and V. I. Klimov, Phys. Rev. Lett., 2004, 92, 186601 CrossRef CAS.
- O. E. Semonin, J. M. Luther, S. Choi, H.-Y. Chen, J. Gao, A. J. Nozik and M. C. Beard, Science, 2011, 334, 1530–1533 CrossRef CAS PubMed.
- J. Zhang, J. Gao, C. P. Church, E. M. Miller, J. M. Luther, V. I. Klimov and M. C. Beard, Nano Lett., 2014, 14, 6010–6015 CrossRef CAS PubMed.
- X. Wang, G. I. Koleilat, J. Tang, H. Liu, I. J. Kramer, R. Debnath, L. Brzozowski, D. A. R. Barkhouse, L. Levina and S. Hoogland, Nat. Photonics, 2011, 5, 480–484 CrossRef CAS PubMed.
- J. Y. Kim, K. Lee, N. E. Coates, D. Moses, T.-Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222–225 CrossRef CAS PubMed.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C.-C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed.
- P. Peumans and S. R. Forrest, Appl. Phys. Lett., 2001, 79, 126–128 CrossRef CAS PubMed.
- C. J. Brabec, S. E. Shaheen, C. Winder, N. S. Sariciftci and P. Denk, Appl. Phys. Lett., 2002, 80, 1288–1290 CrossRef CAS PubMed.
- R. Koeppe, O. Bossart, G. Calzaferri and N. S. Sariciftci, Sol. Energy Mater. Sol. Cells, 2007, 91, 986–995 CrossRef CAS PubMed.
- A. Morales-Acevedo, Sol. Energy, 2009, 83, 1466–1471 CrossRef CAS PubMed.
- L. Tsakalakos, Mater. Sci. Eng., R, 2008, 62, 175–189 CrossRef PubMed.
- I. J. Kramer, L. Levina, R. Debnath, D. Zhitomirsky and E. H. Sargent, Nano Lett., 2011, 11, 3701–3706 CrossRef CAS PubMed.
- C. M. Evans, L. Guo, J. J. Peterson, S. Maccagnano-Zacher and T. D. Krauss, Nano Lett., 2008, 8, 2896–2899 CrossRef CAS PubMed.
- W. W. Yu, J. C. Falkner, B. S. Shih and V. L. Colvin, Chem. Mater., 2004, 16, 3318–3322 CrossRef CAS.
- Y. Zhang, Q. Dai, X. Li, J. Liang, V. L. Colvin, Y. Wang and W. W. Yu, Langmuir, 2011, 27, 9583–9587 CrossRef CAS PubMed.
- P. Gu, Y. Zhang, Y. Feng, T. Zhang, H. Chu, T. Cui, Y. Wang, J. Zhao and W. Y. William, Nanoscale, 2013, 5, 10481–10486 RSC.
- L. Yan, Y. Zhang, X. Zhang, J. Zhao, Y. Wang, T. Zhang, Y. Jiang, W. Gao, J. Yin and J. Zhao, Nanotechnology, 2015, 26, 135201 CrossRef PubMed.
- X. Zhang, Y. Zhang, L. Yan, C. Ji, H. Wu, Y. Wang, P. Wang, T. Zhang, Y. Wang, T. Cui, J. Zhao and W. W. Yu, J. Mater. Chem. A, 2015, 3, 8501–8507 CAS.
- J. M. Luther, M. Law, M. C. Beard, Q. Song, M. O. Reese, R. J. Ellingson and A. J. Nozik, Nano Lett., 2008, 8, 3488–3492 CrossRef CAS PubMed.
- B.-R. Hyun, Y.-W. Zhong, A. C. Bartnik, L. Sun, H. D. Abruna, F. W. Wise, J. D. Goodreau, J. R. Matthews, T. M. Leslie and N. F. Borrelli, ACS Nano, 2008, 2, 2206–2212 CrossRef CAS PubMed.
- I. Robel, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2007, 129, 4136–4137 CrossRef CAS PubMed.
- B. T. Spann, S. V. Bhat, Q. Nian, K. M. Rickey, G. J. Cheng, X. Ruan and X. Xu, Phys. Chem. Chem. Phys., 2014, 16, 10669–10678 RSC.
- I. Robel, V. Subramanian, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2006, 128, 2385–2393 CrossRef CAS PubMed.
- K. Tvrdy, P. A. Frantsuzov and P. V. Kamat, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 29–34 CrossRef CAS PubMed.
- J. J. Choi, Y.-F. Lim, M. E. B. Santiago-Berrios, M. Oh, B.-R. Hyun, L. Sun, A. C. Bartnik, A. Goedhart, G. G. Malliaras and H. D. Abruna, Nano Lett., 2009, 9, 3749–3755 CrossRef CAS PubMed.
- J. Y. Kim, S. H. Kim, H. H. Lee, K. Lee, W. Ma, X. Gong and A. J. Heeger, Adv. Mater., 2006, 18, 572–576 CrossRef CAS PubMed.
- G. H. Kim, B. Walker, H. B. Kim, J. Y. Kim, E. H. Sargent and J. Park, Adv. Mater., 2014, 26, 3321–3327 CrossRef CAS PubMed.
- J. Tang, L. Brzozowski, D. A. R. Barkhouse, X. Wang, R. Debnath, R. Wolowiec, E. Palmiano, L. Levina, A. G. Pattantyus-Abraham and D. Jamakosmanovic, ACS Nano, 2010, 4, 869–878 CrossRef CAS PubMed.
- J. M. Luther, M. Law, Q. Song, C. L. Perkins, M. C. Beard and A. J. Nozik, ACS Nano, 2008, 2, 271–280 CrossRef CAS PubMed.
- M.-J. Choi, J. Oh, J.-K. Yoo, J. Choi, D. M. Sim and Y. S. Jung, Energy Environ. Sci., 2014, 7, 3052–3060 CAS.
| This journal is © The Royal Society of Chemistry 2015 |



