DOI:
10.1039/C5RA10675C
(Communication)
RSC Adv., 2015,
5, 71022-71029
Reinvestigation of the reaction between 1,3-diketones and 2-hydroxyarylaldehydes: a short, atom-economical entry to tetrahydroxanthenones†
Received
5th June 2015
, Accepted 5th August 2015
First published on 5th August 2015
Abstract
A short and facile access to novel functionalized tetrahydroxanthenones via a DABCO-promoted tandem Knoevenagel condensation/hemiketalisation process from easily accessible substrates in a high yield. Tetrahydroxanthenones provide access to the stereo-controlled synthesis of triades or tetrades, which represent privileged structural motifs. Disubstituted tetrahydroxanthenones are generated as a mixture of diastereomers, favoring the formation of selective diastereomers. Unsymmetrical cyclic diketones give mixtures of regio isomers, favoring the formation of sterically less-hindered products.
Introduction
The structural feature of tricyclic xanthenone is found in many natural products,1 especially in a wide variety of fungal metabolites such as secalonic acids, diversonol, beticolins, simaomicins, phomoxanthones and rugulotrosins; most of these show very interesting biological activities (Fig. 1). The secalonic acids exhibit toxic,2 anti-HIV,3 phytotoxic,4 antibacterial,5 and mutagenic6 properties, along with fetotoxic and teratogenic actions.7 To date, only a few syntheses of natural products containing tetrahydroxanthone units have been reported.8 A literature survey revealed that the reaction of salicylaldehyde with a cyclic 1,3-diketone in the presence of various catalysts, such as 2,4,6-trichloro-1,3,5-triazine,9 triethylbenzylammonium chloride (TEBA),10 1-butyl-3-methylimidazolium hydrogen sulfate ([bmim]HSO4),11 cerium(III) chloride heptahydrate (CeCl3·7H2O),12 bulk/nano-ZnAl2O4,13 L-proline,14 cellulose sulfuric acid (biopolymer-based solid acid catalyst),15 silica supported zirconium hydrogen sulfate (Zr(HSO4)4/SiO2),16 toluene-4-sulfonic acid (PTSA)17 and acetic acid,18 leads to 9-substituted tetrahydroxanthenes. Recently, Stefan Brase synthesized 4-hydroxy substituted xanthenones by a three step process.19 Subsequently, Miyabe reported the synthesis of 4-hydroxy substituted xanthenones20 by a coupling reaction induced by the insertion of arynes into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of formamide (Scheme 1). Both the methods involved multiple steps and difficulties in the preparation of starting materials.
O bond of formamide (Scheme 1). Both the methods involved multiple steps and difficulties in the preparation of starting materials.
 |
| | Fig. 1 Some biologically active tetrahydroxanthenones. | |
 |
| | Scheme 1 Previous syntheses of 4-hydroxy xanthenones. | |
Atom economy is one of the major green chemistry principles and involves maximum incorporation of starting materials or reagents into the final product. It is essentially pollution prevention at the molecular level. Atom economy is an important development that goes beyond the traditionally taught concept of percent yield. In recent years, domino reactions21 have attracted more attention in the fields of pharmaceutical and organic chemistry because they allow efficient access to highly functionalized scaffolds, which occur in a variety of natural compounds with biological activities.22
The main advantages of a cascade reaction in organic synthesis are that the reaction is often fast due to its intramolecular nature, it is clean, displays high atom economy, does not involve workup and isolation of many intermediates, and effectively adds considerable complexity in one step. 1,4-Diazabicyclo [2.2.2]octane (DABCO) has emerged as an efficient organic base, which has been successfully used for various organic reactions, such as cross-coupling reactions23 and cyclization of o-alkynylaryl isocyanides.24 To expand the xanthenone library, there is a need to develop a simple method to access cluster molecules using readily available substrates. In continuation of our ongoing research25 on the development of new methods, herein we wish to report a convenient, practical, efficient and cascade synthesis of diversely functionalized xanthenones by the reaction of 2-hydroxy benzaldehyde with cyclic 1,3-diketone in the presence of DABCO (Scheme 2).
 |
| | Scheme 2 DABCO-catalyzed synthesis of new functionalized xanthenones. | |
Results and discussion
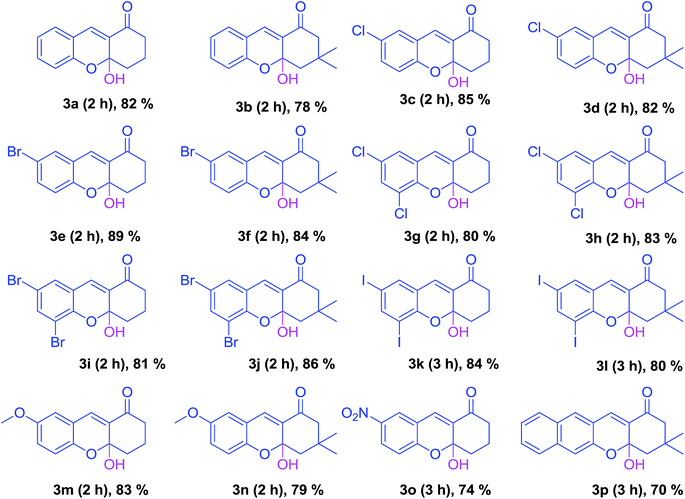
Initially, the reaction between salicylaldehyde (1a) and 1,3-cyclohexanedione (2a) was tested without any catalyst in water, but reaction did not occur even at 100 °C. During the optimization process, we employed different base catalysts such as potassium carbonate (K2CO3), cesium carbonate (Cs2CO3), (1,8-diazabicyclo[5.4.0]undec-7-ene) DBU, pyridine (C5H5N), 2,6-lutidine, triethylamine (TEA), DABCO, triphenylphospine (PPh3), quinuclidine and 4-dimethylaminopyridine (DMAP) and made changes to the ratios of the base at room temperature. However, the reaction gave encouraging results with DABCO (Table 1). Among the different molar ratios (5%, 10%, 15%, 20%, 25%, 30%, and 35%) of DABCO, 5 mol% of catalyst was found to be suitable for the synthesis of the desired xanthenones (Table 2). On the basis of the spectral data, the structure of 4-hydroxy tetrahydroxanthenone was assigned to the product by X-ray crystal structure determination (Fig. 2). THF was the choice of solvent among the screened solvents such as H2O, MeOH, THF, CH3CN, DCM, toluene and DMF (see ESI†). It was concluded that 5 mol% of DABCO in THF gave 4-hydroxy tetrahydroxanthenones, whereas 30 mol% DABCO with 2 equivalents of diketone in THF gave the 9-substituted tetrahydroxanthenones in good yield (Table 3).
Table 1 Results of optimization experimentsa

|
| Entry |
Catalyst |
Solvent |
Time (h) |
Yieldb (%) |
| Reaction conditions: salicylaldehyde (1 mmol), cyclohexane, 1,3-dione (1 mmol) and catalyst stirred in appropriate solvent. Isolated yield of pure product. Trace amount of product. Equiv.: equivalent. |
| 1 |
— |
Water |
12 |
—c |
| 2 |
K2CO3 (1 equiv.) |
DMF |
12 |
10 |
| 3 |
Cs2CO3 (1 equiv.) |
DMF |
12 |
17 |
| 4 |
DBU (10 mol%) |
THF |
5 |
12 |
| 5 |
DBU (5 mol%) |
THF |
12 |
36 |
| 6 |
C5H5N (5 mol%) |
DCM |
12 |
30 |
| 7 |
2,6-Lutidine (10 mol%) |
THF |
12 |
23 |
| 8 |
NEt3 (10 mol%) |
THF |
12 |
39 |
| 9 |
DABCO (5 mol%) |
THF |
2 |
82 |
| 10 |
PPh3 (10 mol%) |
THF |
12 |
49 |
| 11 |
Quinuclidine (5 mol%) |
THF |
12 |
21 |
| 12 |
DMAP (5 mol%) |
DCM |
12 |
16 |
Table 2 Screening of substrates for the synthesis of functionalized xanthenonesa,b

|
| Salicylaldehyde (1 mmol), 1,3-diketone (1 mmol), and DABCO (5 mol%), were added to 2 mL of THF and stirred for 2–3 h. Isolated yield of the pure product. |
 |
 |
| | Fig. 2 X-ray ORTEP diagram of compound 3p. | |
Table 3 Screening of different molar ratios of DABCOa

|
| Entry |
DABCO mol ratio (%) |
Time (h) |
Yieldb 3a (%) |
Yieldc 6a (%) |
| Reaction conditions: salicylaldehyde (1 mmol), cyclohexane, 1,3-dione (1 mmol) and DABCO (mol%) stirred in THF. Isolated yield of pure product (3a). Isolated yield of pure product (Table 6, 6a). Trace amount of product. |
| 1 |
5 mol% |
2 |
82 |
—d |
| 2 |
10 mol% |
3 |
65 |
10 |
| 3 |
15 mol% |
3 |
51 |
25 |
| 4 |
20 mol% |
3 |
29 |
45 |
| 5 |
25 mol% |
3 |
10 |
66 |
| 6 |
30 mol% |
3 |
—d |
85 |
Furthermore, we studied the electronic effect of the substituents on the reaction of aldehyde with symmetric 1,3-diketone. Simple 2-hydroxy benzaldehyde without any substitution gave the desired product in a moderate yield (Table 2, 3a).
A salicylaldehyde bearing halogen (Cl, Br, and I) and a methoxy substituent produced the desired product in good yield. The reaction of a salicylaldehyde having an electron withdrawing substituent (NO2) proceeded slowly and furnished the corresponding product in a low yield. Later, we turned our attention to the reaction of unsymmetric cyclic diketones, which gave regioselective and diastereoselective products. The reaction of 4,4-dimethylcyclohexane-1,3-dione with salicylaldehyde gave a mixture of products (Table 4, 4a and 4a′). The xanthenone (4a), which presents less steric hindrance, was obtained as a major product, whereas the other regioisomer with two methyl groups and a hydroxy group possessed more steric hindrance, which leads to the formation of a minor product (4a′). The electronic effect was also studied and it was found that salicylaldehyde without any substitution gave the desired product (Table 4, 4a) in moderate yield with good regioselectivity. However, 3,5-dihalogen substituted aldehydes reacted relatively slowly and furnished the desired product in a good yield with less regio selectivity (4d) in comparison with simple salicylaldehyde. Furthermore, we studied the reactivity of 5-phenylcyclohexane-1,3-dione in reactions with salicylaldehydes and noticed that a simple salicylaldehyde gave the desired xanthenone in moderate yield with good stereo selectivity (Table 5, 5a). However, halogen substituted aldehydes produced a good yield with less stereo selectivity (5e). The ratios of individual products were found by NMR spectroscopy.
Table 4 Screening of substrates for the regioselective-synthesis of functionalized xanthenonesa,b

|
| Salicylaldehyde (1 mmol), 1,3-diketone (1 mmol), and DABCO (5 mol%) were added to 2 mL THF and stirred for 2–3 h. Isolated yield of mixture of pure products. |
 |
Table 5 Screening of substrates for the synthesis of functionalized xanthenonesa,b

|
| Salicylaldehyde (1 mmol), 1,3-diketone (1 mmol), and DABCO (5 mol%) were added to 2 mL of THF and stirred for 2–3 h. Isolated yield of the mixture of products. |
 |
Table 6 Screening of substrates for the synthesis of hexahydro xanthenesab

|
| Salicylaldehyde (1 mmol), 1,3-diketone (2 mmol), and DABCO (30 mol%) were added to 2 mL of THF and stirred for 8–10 h. Isolated yield of the pure product. |
 |
Plausible mechanism
Initially, diketone reacts with salicylaldehyde in the presence of DABCO to form a Knoevenagel condensed product (B). In the presence of 5 mol% of catalyst, this intermediate undergoes cyclisation with phenolic –OH to give 4-hydroxy tetrahydro xanthenone (3a). However, with 30 mol% of DABCO, the formed condensed product immediately reacts with available diketone to form an intermediate (C), which undergoes cyclisation by eliminating a water molecule to give 9-substituted tetrahydroxanthenone (6a) (Scheme 3).
 |
| | Scheme 3 Plausible mechanistic pathway. | |
Conclusion
We have developed a convenient, practical and atom-economical DABCO-catalyzed synthesis of new functionalized xanthenones. The structure of tetrahydroxanthenones offers various possibilities for further functionalizations. Simple reaction conditions and easy isolation of the products are the advantages of the present method.
Experimental section
General information: salicylaldehydes, β-diketones, DABCO and all other solvents were purchased from Sigma Aldrich and Alpha Aesar Company and were used without further purification. All the 1H and 13C NMR spectra were recorded in deuterated chloroform CDCl3 or CDCl3 + DMSO (deuterated dimethyl sulfoxide) (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) on Avance 300 or Avance 500 spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) relative to residual chloroform (CHCl3) (1H: δ 7.26 ppm, 13C: δ 77.00 ppm) as an internal reference. Coupling constants (J) are reported in Hertz (Hz). Peak multiplicity is indicated as follows: s-singlet, d-doublet, t-triplet, q-quartet, m-multiplet and dd-doublet of doublet. All the signals in 13C NMR spectra appeared as singlets and we have performed 13C NMR using CPD (composite pulse decoupling) method. Melting points were measured on a BUCHI melting point machine. IR spectra were recorded on a Thermo Nicolet FT/IR-5700 spectrometer. Mass spectra were recorded using a Waters mass spectrometer. High resolution mass spectra (HRMS) were recorded using an Applied Bio-Sciences HRMS spectrometer at the National Center for Mass Spectroscopy-IICT.
4) on Avance 300 or Avance 500 spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) relative to residual chloroform (CHCl3) (1H: δ 7.26 ppm, 13C: δ 77.00 ppm) as an internal reference. Coupling constants (J) are reported in Hertz (Hz). Peak multiplicity is indicated as follows: s-singlet, d-doublet, t-triplet, q-quartet, m-multiplet and dd-doublet of doublet. All the signals in 13C NMR spectra appeared as singlets and we have performed 13C NMR using CPD (composite pulse decoupling) method. Melting points were measured on a BUCHI melting point machine. IR spectra were recorded on a Thermo Nicolet FT/IR-5700 spectrometer. Mass spectra were recorded using a Waters mass spectrometer. High resolution mass spectra (HRMS) were recorded using an Applied Bio-Sciences HRMS spectrometer at the National Center for Mass Spectroscopy-IICT.
General procedure
In a typical experiment, the salicylaldehyde (1 mmol), cyclohexane 1,3-dione (1 mmol), and DABCO (5 mol%) in THF (2 mL) were placed in a 10 mL round-bottomed flask and stirred at room temperature for 2 h. After the completion of the reaction (monitored by TLC), the solvent was removed under reduced pressure and the crude product was purified by column chromatography using ethyl acetate/hexane. All the compounds were characterized by (NMR, Mass, and IR) spectral data.
Spectral data of all compounds
4a-Hydroxy-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3a). White solid; mp 155–157 °C; IR: vmax 3243, 2956, 2927, 1627, 1604, 1561, 1454, 1294, 1172, 979, 842, 761 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.47 (s, 1H), 7.28–7.37 (m, 2H), 6.97–7.07 (m, 2H), 6.34 (s, 1H), 2.43–2.68 (m, 2H), 2.28–2.42 (m, 1H), 2.07–2.23 (m, 2H), 1.91–2.04 (m, 1H), 13C NMR (75 MHz, CDCl3 + DMSO): δ 197.2, 152.7, 130.9, 130.5, 128.9, 128.6, 120.9, 119.7, 116.2, 95.9, 38.3, 35.4, 17.5; m/z (ESI); 199 [M − H2O]H+.
4a-Hydroxy-3,3-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3b). White solid; mp 128–130 °C; IR: vmax 3246, 2958, 2915, 1624, 1609, 1559, 1457, 1293, 1165, 968, 844, 768 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.43 (s, 1H), 7.27–7.39 (m, 2H), 6.96–7.04 (m, 2H), 6.38 (s, 1H), 2.87 (s, 3H), 2.37–2.60 (m, 2H), 2.19–2.31 (m, 2H), 1.18 (s, 3H), 1.10 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 198.0, 152.7, 131.3, 129.6, 129.4, 129.0, 121.3, 120.1, 116.7, 96.3, 52.3, 48.7, 31.3, 30.2, 27.9; m/z (ESI); 227 [M − H2O]H+. HRMS calcd for C15H15O2: 227.10666, found: 227.10576.
7-Chloro-4a-hydroxy-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3c). White solid; mp 168–170 °C; IR: vmax 3346, 2959, 1720, 1670, 1607, 1547, 1469, 1390, 1259, 1133, 1059, 931, 819 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.46 (d, J = 2.3 Hz, 1H), 7.39 (dd, J1 = 2.3 Hz, J2 = 8.7 Hz, 1H), 6.91 (d, J = 8.69 Hz, 1H), 6.63 (s, 1H), 2.89 (s, 1H), 2.56–2.69 (m, 1H), 2.27–2.54 (m, 2H), 1.92–2.24 (m, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.5, 151.4, 131.2, 130.5, 126.7, 121.4, 117.8, 112.3, 95.8, 38.0, 34.9, 17.2; m/z (ESI); 233 [M − H2O]H+. HRMS calcd for C13H10O2Cl: 233.03638, found: 233.03538.
7-Chloro-4a-hydroxy-3,3-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3d). White solid; mp 131–133 °C; IR: vmax 3337, 2943, 1717, 1664, 1507, 1445, 1383, 1261, 1147, 1052, 928, 814 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.41 (s, 1H), 7.34 (d, J = 1.9 Hz, 1H), 7.30 (dd, J1 = 2.4 Hz, J2 = 8.7 Hz, 1H), 6.99 (d, J = 8.7 Hz, 1H), 2.99 (s, 1H), 2.49–2.54 (m, 1H), 2.28–2.37 (m, 3H), 1.18 (s, 3H), 1.12 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 197.1, 150.8, 130.3, 130.1, 127.7, 126.8, 125.1, 121.1, 90.0, 51.9, 47.9, 30.8, 29.7, 27.1; m/z (ESI); 261 [M − H2O]H+. HRMS C15H15O2Cl: found: 261.08969.
7-Bromo-4a-hydroxy-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3e). White solid; mp 172–174 °C; IR: vmax 3261, 2976, 2943, 1658, 1605, 1547, 1463, 1284, 1190, 1011, 928, 813 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.45 (s, 1H), 7.34 (d, J = 2.6 Hz, 1H), 7.30 (dd, J1 = 2.4 Hz, J2 = 8.7 Hz, 1H), 6.99 (d, J = 8.7 Hz, 1H), 2.97 (s, 1H), 2.49–2.54 (m, 1H), 2.67–2.73 (m, 1H), 2.32–2.42 (m, 1H), 2.10–2.26 (m, 2H), 1.99–2.05 (m, 1H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.6, 151.4, 132.9, 131.1, 130.5, 126.8, 121.3, 117.9, 112.3, 95.8, 38.0, 34.9, 17.2; m/z (ESI); 277 [M − H2O]H+.
7-Bromo-4a-hydroxy-3,3-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3f). White solid; mp 135–137 °C; IR: vmax 3257, 2980, 2953, 1660, 1602, 1549, 1466, 1282, 1189, 1000, 914, 817 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.49 (d, J = 2.3 Hz, 1H), 7.30 (dd, J1 = 2.4 Hz, J2 = 8.7 Hz, 1H), 6.93 (d, J = 8.4 Hz, 1H), 3.00 (s, 1H), 2.49–2.54 (m, 1H), 2.28–2.37 (m, 3H), 1.18 (s, 3H), 1.12 (s, 3H); 13C NMR (75 MHz, CDCl3 + CDCl3 + DMSO): δ 197.1, 150.8, 130.3, 130.1, 127.7, 126.8, 125.1, 121.1, 90.0, 51.9, 47.9, 30.8, 29.7, 27.1; m/z (ESI); 305 [M − H2O]H+. HRMS calcd for C15H14O2Br: 305.01717, found: 305.01588.
5,7-Dichloro-4a-hydroxy-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3g). White solid; mp 170–172 °C; IR: vmax 3311, 2974, 2952, 2919, 1664, 1601, 1558, 1448, 1339, 1207, 1189, 1026, 984, 763 cm−1; 1H NMR (300 MHz CDCl3 + DMSO): δ 7.42 (s, 1H), 7.40 (d, J = 2.4 Hz, 1H), 7.26 (d, J = 2.4 Hz, 1H), 3.15 (s, 1H), 2.68–2.74 (m, 1H), 2.53–2.58 (m, 1H), 2.27–2.43 (m, 2H), 2.02–2.19 (m, 2H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.9, 147.4, 132.2, 130.4, 126.9, 125.4, 122.2, 122.1, 97.2, 38.5, 35.3, 17.6; m/z (ESI); 267 [M − H2O]H+.
5,7-Dichloro-4a-hydroxy-3,3-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3h). White solid; mp 144–146 °C; IR: vmax 3305, 2972, 2955, 2923, 1660, 1605, 1561, 1456, 1344, 1211, 1186, 1018, 998, 756 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.40 (d, J = 2.4 Hz, 1H), 7.38 (s, 1H), 7.26 (d, J = 2.4 Hz, 1H), 3.18 (s, 1H), 2.50–2.55 (m, 1H), 2.39–2.46 (m, 2H), 2.28–2.33 (m, 1H), 1.19 (s, 3H), 1.13 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 197.3, 147.2, 131.2, 130.3, 126.7, 125.3, 122.4, 122.1, 97.2, 52.2, 47.9, 31.1, 30.1, 27.2; m/z (ESI); 295 [M − H2O]H+. HRMS for C15H14O2Cl2: 295.02727.
5,7-Dibromo-4a-hydroxy-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3i). White solid; mp 158–160 °C; IR: vmax 3308, 3143, 2956, 2887, 1681, 1594, 1540, 1429, 1265, 1193, 985, 858 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.65 (d, J = 2.1 Hz, 1H), 7.50 (s, 1H), 7.44 (d, J = 2.3 Hz, 1H), 6.92 (d, J = 0.9 Hz, 1H), 2.56–2.71 (m, 2H), 1.94–2.44 (m, 4H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.6, 148.7, 135.5, 132.0, 130.1, 126.5, 125.5, 112.4, 111.0, 97.1, 38.3, 35.0, 17.3; m/z (ESI); 355 [M − H2O]H+. HRMS calcd for C13H9O2Br2: 354.89638, found: 354.89503.
5,7-Dibromo-4a-hydroxy-3,3-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3j). White solid; mp 151–154 °C; IR: vmax 3311, 3150, 2961, 2882, 1685, 1598, 1539, 1439, 1276, 1200, 1156, 978, 947, 864 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.70 (d, J = 2.3 Hz, 1H), 7.45 (d, J = 2.3 Hz, 1H), 7.37 (s, 1H), 3.04 (s, 1H), 2.27–2.59 (m, 4H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 195.4, 147.0, 133.7, 129.8, 129.6, 129.1, 124.7, 121.7, 110.7, 109.4, 95.8, 50.5, 45.8, 29.4, 28.5, 25.4; m/z (ESI); 384 [M − H2O]H+. HRMS calcd for C15H13O2Br2: 382.92768, found: 382.92612.
4a-Hydroxy-5,7-diiodo-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3k). White solid; mp 174–176 °C; IR: vmax 3229, 2950, 2867, 1666, 1609, 1525, 1434, 1317, 1282, 1155, 1098, 981, 856 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 8.00 (s, 1H), 7.62 (s, 1H), 6.95 (s, 1H), 2.51–2.68 (m, 2H), 1.88–2.44 (m, 4H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.5, 151.8, 146.5, 136.8, 131.8, 126.3, 122.3, 97.3, 85.9, 83.1, 38.2, 34.9, 17.3; m/z (ESI); 451 [M − H2O]H+. HRMS calcd for C13H9O2I2: 450.86864, found: 450.86643.
4a-Hydroxy-5,7-diiodo-3,3-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3l). White solid; mp 146–148 °C; IR: vmax 3234, 2946, 2863, 1662, 1605, 1529, 1428, 1314, 1278, 1158, 1094, 983, 850 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 8.00 (s, 1H), 7.63 (s, 1H), 6.93 (s, 1H), 3.04 (s, 1H), 2.40–2.61 (m, 2H), 2.22–2.34 (m, 2H), 1.17 (s, 3H), 1.11 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.8, 151.6, 146.4, 136.8, 130.7, 122.5, 97.3, 85.9, 83.0, 52.0, 47.7, 30.9, 29.9, 27.0; m/z (ESI); 479 [M − H2O]H+. HRMS calcd for C15H13O2I2: 478.89754, found: 478.89719.
4a-Hydroxy-7-methoxy-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3m). White solid; mp 128–130 °C; IR: vmax 3305, 3011, 2938, 1669, 1610, 1564, 1487, 1239, 1171, 1059, 980, 865 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.43 (d, J = 2.3 Hz, 1H), 6.82–6.99 (m, 2H), 6.02–6.09 (m, 1H), 3.80 (s, 3H), 1.83–2.64 (m, 6H); 13C NMR (75 MHz, CDCl3): δ 197.2, 153.5, 146.9, 131.5, 128.4, 120.5, 117.9, 117.1, 113.1, 96.1, 55.4, 38.4, 35.4, 17.8; m/z (ESI); 229 [M − H2O]H+. HRMS for C13H13O3: found: 229.08490.
4a-Hydroxy-7-methoxy-3,3-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3n). White solid; mp 132–134 °C; IR: vmax 3311, 3005, 2941, 1672, 1607, 1573, 1484, 1246, 1178, 1049, 977, 862 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.44 (s, 1H), 6.92–6.99 (m, 2H), 6.85 (d, J = 2.9 Hz, 1H), 3.79 (s, 3H), 3.09 (s, 1H), 2.47–2.52 (m, 1H), 2.26–2.37 (m, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 198.1, 154.4, 146.4, 130.0, 129.7, 120.1, 119.1, 118.2, 112.7, 96.4, 55.7, 52.5, 48.8, 30.6, 28.0; m/z (ESI); 257 [M − H2O]H+. HRMS calcd for C16H17O3: 257.11482, found: 257.11599.
4a-Hydroxy-7-nitro-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 3o). White solid; mp 164–166 °C; IR: vmax 3396, 3092, 2949, 2879, 1674, 1605, 1569, 1529, 1346, 1271, 1210, 1161, 974, 841 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 8.28 (d, J = 2.8 Hz, 1H), 8.19 (dd, J1 = 2.6 Hz, J2 = 8.9 Hz, 1H), 7.47 (s, 1H), 7.10–7.15 (m, 2H), 2.51–2.73 (m, 2H), 2.32–2.47 (m, 1H), 1.95–2.27 (m, 3H), 3.00 (s, 1H), 2.49–2.54 (m, 1H), 2.28–2.37 (m, 3H), 1.18 (s, 3H), 1.12 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.7, 157.7, 141.3, 132.3, 126.6, 125.8, 124.4, 119.8, 117.0, 97.5, 38.4, 35.2, 17.5; m/z (ESI); 244 [M − H2O]H+.
4a-Hydroxy-3,3-dimethyl-2,3,4,4a-tetrahydro-1H-benzo[b]xanthen-1-one (Table 2, 3p). White solid; mp 124–126 °C; IR: vmax 3280, 2966, 2929, 1628, 1576, 1476, 1373, 1289, 1242, 964, 823 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 8.26 (s, 1H), 8.16 (d, J = 8.5 Hz, 1H), 7.77–7.88 (m, 2H), 7.55–7.63 (m, 1H), 7.41–7.48 (m, 1H), 7.21–7.28 (m, 1H), 3.20 (s, 1H), 2.28–2.57 (m, 4H), 1.21 (s, 3H), 1.13 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 197.2, 151.5, 131.6, 128.4, 127.9, 127.1, 126.8, 124.7, 123.6, 120.9, 117.6, 113.0, 96.1, 51.9, 48.2, 30.8, 29.9, 27.3; m/z (ESI); 277 [M − H2O]H+. HRMS calcd for C19H18O3Na: 317.11482, found: 317.11330.
4a-Hydroxy-2,2-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 4, 4a). White solid; mp 133–135 °C; IR: vmax 3237, 2948, 2913, 1625, 1611, 1546, 1462, 1284, 1179, 963, 851, 764 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.42 (s, 1H), 7.27–7.37 (m, 2H), 6.96–7.03 (m, 2H), 6.59 (d, J = 3.4 Hz, 1H), 2.57–2.61 (m, 1H), 2.34–2.38 (m, 1H), 2.02–2.15 (m, 1H), 1.63–1.73 (m, 1H), 1.19 (s, 3H), 1.12 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 202.2, 152.4, 130.8, 129.6, 129.3, 128.7, 120.9, 119.7, 116.1, 95.8, 42.1, 32.3, 32.1, 25.0, 24.9; m/z (ESI); 227 [M − H2O]H+. HRMS calcd for C15H15O2: 227.10666, found: 227.10563.
7-Chloro-4a-hydroxy-2,2-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 2, 4b). White solid; mp 161–163 °C; IR: vmax 3311, 3056, 2964, 2938, 2861, 1663, 1624, 1559, 1455, 1387, 1180, 1050, 930, 818, 750 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.34 (s, 1H), 7.32 (d, J = 2.5 Hz, 2H), 7.25 (dd, J1 = 2.5 Hz, J2 = 8.69 Hz, 1H), 6.95 (d, J = 8.69 Hz, 2H), 6.67 (s, 1H), 2.24–2.40 (m, 2H), 2.01–2.13 (m, 1H), 1.63–1.73 (m, 1H), 1.19 (s, 3H), 1.12 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 201.4, 150.5, 130.3, 127.3, 127.2, 124.7, 120.7, 117.1, 95.7, 41.7, 31.7, 31.4, 24.4; m/z (ESI); 261 [M − H2O]H+. HRMS for C15H15O2Cl: found: 261.08969.
7-Bromo-4a-hydroxy-2,2-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 4, 4c). White solid; mp 155–157 °C; IR: vmax 3269, 2968, 2924, 2861, 1673, 1620, 1556, 1472, 1245, 1152, 1048, 943, 816 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.47 (d, J = 2.5 Hz, 1H), 7.38 (dd, J1 = 2.5 Hz, J2 = 8.7 Hz, 1H), 7.33 (s, 1H), 6.91 (d, J = 2.5 Hz, 1H), 6.74 (s, 1H), 2.29–2.37 (m, 2H), 2.00–2.13 (m, 1H), 1.63–1.73 (m, 1H), 1.19 (s, 3H), 1.12 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 201.9, 151.3, 132.9, 130.5, 127.7, 121.6, 117.9, 112.5, 96.1, 42.1, 32.1, 31.8, 24.8; m/z (ESI); 305 [M − H2O]H+. HRMS calcd for C15H14O2Br: 305.01717, found: 305.01565.
5,7-Dichloro-4a-hydroxy-2,2-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 4, 4d). White solid; mp 137–139 °C; IR: vmax 3312, 2978, 2946, 2924, 1659, 1607, 1562, 1445, 1333, 1226, 1191, 1012, 988, 751 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.01 (d, J = 8.3 Hz, 1H), 7.60 (d, J = 8.3 Hz, 1H), 6.64 (s, 1H), 2.41–2.61 (m, 2H), 1.99–2.16 (m, 1H), 1.65–1.77 (m, 1H), 1.20 (s, 3H), 1.12 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 202.3, 147.1, 131.6, 131.5, 129.5, 129.1, 127.3, 127.2, 97.5, 43.1, 32.7, 25.3, 25.2; m/z (ESI); 295 [M − H2O]H+. HRMS calcd for C15H13O2Cl2: 295.02871, found: 295.02727.
5,7-Dibromo-4a-hydroxy-2,2-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 4, 4e). White solid; mp 144–146 °C; IR: vmax 165–167 °C; IR: vmax 3317, 3148, 2953, 2874, 1687, 1589, 1536, 1433, 1264, 1187, 1151, 945, 861 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.65 (s, 1H), 7.42 (s, 1H), 7.32 (s, 1H), 6.73 (s, 1H), 2.48–2.62 (m, 2H), 2.01–2.18 (m, 1H), 1.64–1.76 (m, 1H), 1.20 (s, 3H), 1.12 (s, 3H); m/z (ESI); 383 [M − H2O]H+.
4a-Hydroxy-5,7-diiodo-2,2-dimethyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 4, 4f). White solid; mp 163–165 °C; IR: vmax 3237, 2951, 2864, 1668, 1609, 1523, 1422, 1315, 1282, 1156, 1098, 981, 855 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.35–7.39 (m, 1H), 7.31–7.35 (m, 2H), 7.09 (s, 1H), 2.29–2.56 (m, 2H), 1.94–2.12 (m, 1H), 1.65–1.76 (m, 1H), 1.18 (s, 3H), 1.11 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 201.8, 147.0, 131.4, 129.9, 127.3, 126.4, 125.1, 122.1, 121.8, 97.1, 42.3, 32.1, 31.8, 24.8, 24.7; m/z (ESI); 479 [M − H2O]H+.
4a-Hydroxy-2,2-dimethyl-2,3,4,4a-tetrahydro-1H-benzo[b]xanthen-1-one (Table 4, 4g). White solid; mp 161–163 °C; IR: vmax 3280, 2966, 2929, 1628, 1576, 1476, 1373, 1289, 1123, 964, 823 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 8.23 (s, 1H), 8.16 (d, J = 1.7 Hz, 1H), 7.77–7.86 (m, 2H), 7.53–7.61 (m, 1H), 7.38–7.45 (m, 1H), 7.23 (d, J = 1.7 Hz, 1H), 6.59 (s, 1H), 2.55–2.60 (m, 1H), 2.41–2.46 (m, 1H), 2.08–2.20 (m, 1H), 1.68–1.76 (m, 1H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 202.2, 151.7, 131.7, 130.5, 128.6, 128.1, 127.4, 126.9, 125.7, 123.7, 120.9, 117.7, 112.9, 96.2, 42.0, 32.4, 32.1, 25.2; m/z (ESI); 277 [M − H2O]H+.
4a-Hydroxy-3-phenyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 5, 5a). White solid; mp 146–148 °C; IR: vmax 3389, 3030, 2922, 2852, 1669, 1604, 1558, 1453, 1297, 1261, 1200, 1163, 1094, 970, 765 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.56 (s, 1H), 7.20–7.44 (s, 7H), 6.99–7.08 (m, 2H), 3.72 (s, 1H), 3.44–3.59 (m, 1H), 2.77–2.90 (m, 1H), 2.62–2.74 (m, 1H), 2.40–2.57 (m, 2H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.3, 152.8, 142.8, 131.2, 129.6, 129.1, 128.2, 126.1, 121.2, 119.8, 116.4, 95.7, 46.4, 42.6, 35.4; m/z (ESI); 275 [M − H2O]H+. HRMS for C19H15O2: found: 275.10542.
7-Chloro-4a-hydroxy-3-phenyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 5, 5b). White solid; mp 159–161 °C; IR: vmax 3390, 3030, 2929, 1675, 1617, 1555, 1470, 1283, 1200, 1162, 1081, 967, 824 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.50 (s, 1H), 7.22–7.46 (m, 7H), 3.46–3.60 (m, 1H), 3.38 (s, 1H), 2.83–3.03 (m, 1H), 2.43–2.73 (m, 3H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 195.6, 150.9, 142.3, 130.3, 130.2, 127.8, 127.7, 127.2, 125.8, 125.2, 120.8, 117.5, 95.6, 46.0, 42.1, 34.9; m/z (ESI); 309 [M − H2O]H+. HRMS for C19H14O2Cl: found: 309.06605.
7-Bromo-4a-hydroxy-3-phenyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 5, 5c). White solid; mp 154–156 °C; IR: vmax 3384, 3036, 2937, 1683, 1613, 1552, 1466, 1278, 1201, 1167, 1078, 965, 826 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.50 (d, J = 2.1 Hz, 1H), 7.43 (s, 1H), 7.32–7.41 (m, 2H), 7.22–7.29 (m, 2H), 6.98 (s, 1H), 6.93 (d, J = 8.3 Hz, 1H), 3.45–3.59 (m, 1H), 2.95 (s, 1H), 2.78–2.89 (m, 1H), 2.65–2.74 (m, 1H), 2.37–2.61 (m, 2H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 196.2, 151.8, 142.7, 136.6, 131.1, 130.6, 128.3, 127.7, 126.3, 126.2, 121.8, 118.4, 113.1, 96.0, 46.5, 42.6, 35.4; m/z (ESI); 353 [M − H2O]H+. HRMS calcd for C19H14O2Br: 353.01717, found: 353.01595.
5,7-Dichloro-4a-hydroxy-3-phenyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 5, 5d). White solid; mp 168–170 °C; IR: vmax 3392, 3035, 2926, 1678, 1614, 1557, 1471, 1285, 1204, 1169, 1083, 962, 828 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.50 (s, 1H), 7.43 (d, J = 2.4 Hz, 1H), 7.35–7.40 (m, 2H), 7.26–7.31 (m, 4H), 3.50–3.58 (m, 1H), 3.11 (s, 1H), 2.91–2.97 (m, 1H), 2.75–2.80 (m, 1H), 2.53–2.64 (m, 2H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 195.7, 147.2, 142.3, 131.2, 130.2, 128.0, 126.9, 126.1, 126.0, 125.2, 122.0, 121.9, 96.7, 46.2, 42.3, 35.1; m/z (ESI); 343 [M − H2O]H+.
5,7-Dibromo-4a-hydroxy-3-phenyl-2,3,4,4a-tetrahydro-1H-xanthen-1-one (Table 5, 5e). White solid; mp 172–174 °C; IR: vmax 3390, 3030, 2929, 1675, 1617, 1555, 1470, 1283, 1200, 1162, 1081, 967, 824 cm−1; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.53–7.72 (m, 2H), 7.15–7.52 (m, 7H), 2.39–2.86 (m, 5H); 13C NMR (75 MHz, CDCl3 + DMSO): δ 194.7, 147.7, 141.5, 134.5, 130.3, 129.5, 127.1, 125.7, 125.2, 121.8, 111.5, 109.9, 95.9, 45.1, 41.2, 34.2; m/z (ESI); 431 [M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O]H+.
H2O]H+.
4a-Hydroxy-3-phenyl-2,3,4,4a-tetrahydro-1H-benzo[b]xanthen-1-one (Table 5, 5f). White solid; mp 155–157 °C; IR: vmax 3389, 3030, 2922, 2852, 1669, 1604, 1558, 1453, 1297, 1261, 1200, 1163, 1094, 970, 765 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.37 (s, 1H), 8.19 (d, J = 8.3 Hz, 1H), 7.72–7.89 (m, 2H), 7.52–7.64 (m, 1H), 7.22–7.49 (m, 8H), 3.52–3.66 (m, 1H), 3.47 (s, 1H), 2.86–3.00 (m, 1H), 2.66–2.81 (m, 1H), 2.48–2.63 (m, 2H); 13C NMR (75 MHz, CDCl3): δ 196.4, 152.7, 142.8, 131.2, 129.6, 129.2, 129.1, 128.2, 126.1, 121.2, 119.7, 116.4, 95.7, 46.4, 42.6, 35.4; m/z (ESI); 325 [M − H2O]H+.
Acknowledgements
The authors thank CSIR, New Delhi, for financial support as part of XII. Five Year Plan Program under the title ORIGIN (CSC-0108) and Dr A. Kamal, outstanding scientist and Head of MCP Division, for his support and encouragement. LCR and NSK thank CSIR for the award of a fellowship.
References
- S. Bräse, A. Encinas, J. Keck and C. F. Nising, Chem. Rev., 2009, 109, 3903–3990 CrossRef PubMed
 .
. -
(a) C. S. Reddy, A. W. Hayes, W. L. Williams and A. Ciegler, J. Toxicol. Environ. Health, 1979, 5, 1159–1169 CrossRef CAS PubMed
 ;
(b) A. Ciegler, A. W. Hayes and R. F. Vesonder, Appl. Environ. Microbiol., 1980, 39, 285–287 CAS
;
(b) A. Ciegler, A. W. Hayes and R. F. Vesonder, Appl. Environ. Microbiol., 1980, 39, 285–287 CAS  .
. - F. McPhee, P. S. Caldera, G. W. Bemis, A. F. McDonagh, I. D. Kuntz and C. S. Craik, Biochem. J., 1996, 320, 681–686 CrossRef CAS
 .
. - B. H. Wang and G. M. Polya, Planta Med., 1996, 62, 111–114 CrossRef CAS PubMed
 .
. - A. Stoll, J. Renz and A. Brack, Helv. Chim. Acta, 1952, 35, 2022–2034 CrossRef CAS PubMed
 .
. -
(a) F. C. Wehner, P. G. Thiel, S. J. van Rensburg and I. P. C. Demasius, Mutat. Res., 1978, 58, 193–203 CrossRef CAS
 ;
(b) C. S. Reddy, R. V. Reddy, P. K. Chan and A. W. Hayes, J. Environ. Pathol. Toxicol., 1980, 4, 31–37 CAS
;
(b) C. S. Reddy, R. V. Reddy, P. K. Chan and A. W. Hayes, J. Environ. Pathol. Toxicol., 1980, 4, 31–37 CAS  .
. -
(a) C. S. Reddy, R. V. Reddy, A. W. Hayes and A. Ciegler, J. Toxicol. Environ. Health, 1981, 7, 445–455 CrossRef CAS PubMed
 ;
(b) K. Mayura, A. W. Hayes and W. O. Berndt, Toxicology, 1982, 25, 311–322 CrossRef CAS
;
(b) K. Mayura, A. W. Hayes and W. O. Berndt, Toxicology, 1982, 25, 311–322 CrossRef CAS  .
. -
(a) D. L. da Silva, B. S. Terra, M. R. Lage, A. L. T. Gois Ruiz, C. C. da Silva, J. E. de Carvalho, J. W. de Mesquita Carneiro, F. T. Martins, S. A. Fernadese and A. de Fatima, Org. Biomol. Chem., 2015, 13, 3280–3287 RSC
 ;
(b) O. E. Sepelgy, S. Haseloff, S. K. Alamsetti and C. Schneider, Angew. Chem., Int. Ed., 2014, 53, 7923–7927 CrossRef PubMed
;
(b) O. E. Sepelgy, S. Haseloff, S. K. Alamsetti and C. Schneider, Angew. Chem., Int. Ed., 2014, 53, 7923–7927 CrossRef PubMed  ;
(c) K. C. Nicolaou, H. Yu and M. Wartmann, Angew. Chem., Int. Ed., 2005, 117, 766–771 CrossRef PubMed
;
(c) K. C. Nicolaou, H. Yu and M. Wartmann, Angew. Chem., Int. Ed., 2005, 117, 766–771 CrossRef PubMed  ;
(d) E. J. Tisdale, I. Slobodov and E. A. Theodorakis, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12030–12035 CrossRef CAS PubMed
;
(d) E. J. Tisdale, I. Slobodov and E. A. Theodorakis, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12030–12035 CrossRef CAS PubMed  ;
(e) B. Franck, J. Stöckigt, U. Zeidler and G. Franckowiak, Chem. Ber., 1973, 106, 1198–1220 CrossRef CAS PubMed
;
(e) B. Franck, J. Stöckigt, U. Zeidler and G. Franckowiak, Chem. Ber., 1973, 106, 1198–1220 CrossRef CAS PubMed  .
. - P. Zhang, Y. D. Yu and Z. H. Zhang, Synth. Commun., 2008, 38, 4474–4479 CrossRef CAS PubMed
 .
. - X. S. Wang, D. Q. Shi, Y. L. Li, H. Chen, X. Y. Wei and Z. M. Zong, Synth. Commun., 2005, 35, 97–104 CrossRef CAS PubMed
 .
. - H. M. Majid, A. Parvaneh, S. Mina, K. Narges and T. H. Niloofar, Bull. Chem. Soc. Ethiop., 2011, 25, 315–320 Search PubMed
 .
. - G. Sabitha, K. Arundhathi, K. Sudhakar, B. S. Sastry and J. S. Yadav, Synth. Commun., 2008, 38, 3439–3446 CrossRef CAS PubMed
 .
. - M. T. Rajashekhar, U. Balijapalli and S. Kulathu, J. Mol. Catal. A: Chem., 2014, 391, 198–207 CrossRef PubMed
 .
. - P. Davinder, P. Amreeta and N. Mahendra, C. R. Chim., 2013, 16, 1153–1157 CrossRef PubMed
 .
. - B. S. Kuarm, J. V. Madhav, S. V. Laxmi, B. Rajitha, Y. T. Reddy, P. N. Reddy and P. A. Crooks, Synth. Commun., 2011, 41, 1719–1724 CrossRef CAS PubMed
 .
. - A. Shahrzad, Lett. Org. Chem., 2014, 11, 350–355 CrossRef
 .
. - N. Lingaiah, K. Shuklachary, B. Rajashaker and B. Sridhar, Synth. Commun., 2012, 42, 967–974 CrossRef PubMed
 .
. - S. Nagaaki, J. Makoto, S. Takunobu, H. Tomoko, N. Katsumasa, M. Minoru, H. Yuji, S. Aya, A. Makoto, O. Tomoyuki, I. Hisashi, G. Akira, I. Akane, K. Akio and F. Takehiro, J. Med. Chem., 2008, 51, 4765–4770 CrossRef PubMed
 .
. - C. F. Nising, U. K. Ohnemuller, A. Friedrich, B. Lesch, J. Steiner, H. Schnçckel, M. Nieger and S. Brase, Chem.–Eur. J., 2006, 12, 3647–3654 CrossRef CAS PubMed
 .
. - E. Yoshioka, S. Kohtani and H. Miyabe, Angew. Chem., Int. Ed., 2011, 50, 6638–6642 CrossRef CAS PubMed
 .
. -
(a) L. F. Tietze, G. Brasche and K. M. Gericke, in Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006 Search PubMed
 ;
(b) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed
;
(b) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed  ;
(c) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed., 1993, 32, 131–163 CrossRef PubMed
;
(c) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed., 1993, 32, 131–163 CrossRef PubMed  ;
(d) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed., 1993, 105, 137–170 CrossRef CAS PubMed
;
(d) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed., 1993, 105, 137–170 CrossRef CAS PubMed  .
. -
(a) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134–7186 CrossRef CAS PubMed
 ;
(b) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 118, 7292–7344 CrossRef PubMed
;
(b) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 118, 7292–7344 CrossRef PubMed  .
. -
(a) Y. J. Shi, G. Humphrey, P. E. Maligres, R. A. Reamer and J. M. Williams, Adv. Synth. Catal., 2006, 348, 309–312 CrossRef CAS PubMed
 ;
(b) G. L. Zhao and M. Shi, Tetrahedron, 2005, 61, 7277–7288 CrossRef CAS PubMed
;
(b) G. L. Zhao and M. Shi, Tetrahedron, 2005, 61, 7277–7288 CrossRef CAS PubMed  .
. -
(a) Y. L. Shi and M. Shi, Org. Lett., 2005, 7, 3057–3060 CrossRef CAS PubMed
 ;
(b) W. Liu and G. Zhao, Org. Biomol. Chem., 2014, 12, 832–835 RSC
;
(b) W. Liu and G. Zhao, Org. Biomol. Chem., 2014, 12, 832–835 RSC  ;
(c) W. H. Miles, P. G. Gildner, Z. Ahmed and E. M. Cohen, Synthesis, 2010, 23, 3977–3979 CrossRef
;
(c) W. H. Miles, P. G. Gildner, Z. Ahmed and E. M. Cohen, Synthesis, 2010, 23, 3977–3979 CrossRef  .
. -
(a) L. C. Rao, N. S. Kumar, N. J. babu and H. M. Meshram, Tetrahedron Lett., 2014, 55, 5342–5346 CrossRef PubMed
 ;
(b) L. C. Rao, H. M. Meshram, N. S. Kumar, N. N. Rao and N. J. Babu, Tetrahedron Lett., 2014, 55, 1127–1131 CrossRef PubMed
;
(b) L. C. Rao, H. M. Meshram, N. S. Kumar, N. N. Rao and N. J. Babu, Tetrahedron Lett., 2014, 55, 1127–1131 CrossRef PubMed  ;
(c) L. C. Rao, N. S. Kumar, V. Dileepkumar, U. S. N. Murthy and H. M. Meshram, RSC Adv., 2015, 5, 28958–28964 RSC
;
(c) L. C. Rao, N. S. Kumar, V. Dileepkumar, U. S. N. Murthy and H. M. Meshram, RSC Adv., 2015, 5, 28958–28964 RSC  ;
(d) L. C. Rao, N. S. Kumar and H. M. Meshram, Helv. Chim. Acta, 2015, 98, 978–985 CrossRef CAS PubMed
;
(d) L. C. Rao, N. S. Kumar and H. M. Meshram, Helv. Chim. Acta, 2015, 98, 978–985 CrossRef CAS PubMed  ;
(e) L. C. Rao, N. S. Kumar, N. J. Babu and H. M. Meshram, New J. Chem., 2015 10.1039/c5nj01377a
;
(e) L. C. Rao, N. S. Kumar, N. J. Babu and H. M. Meshram, New J. Chem., 2015 10.1039/c5nj01377a  .
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1032788. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra10675c |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of formamide (Scheme 1). Both the methods involved multiple steps and difficulties in the preparation of starting materials.
O bond of formamide (Scheme 1). Both the methods involved multiple steps and difficulties in the preparation of starting materials.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) on Avance 300 or Avance 500 spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) relative to residual chloroform (CHCl3) (1H: δ 7.26 ppm, 13C: δ 77.00 ppm) as an internal reference. Coupling constants (J) are reported in Hertz (Hz). Peak multiplicity is indicated as follows: s-singlet, d-doublet, t-triplet, q-quartet, m-multiplet and dd-doublet of doublet. All the signals in 13C NMR spectra appeared as singlets and we have performed 13C NMR using CPD (composite pulse decoupling) method. Melting points were measured on a BUCHI melting point machine. IR spectra were recorded on a Thermo Nicolet FT/IR-5700 spectrometer. Mass spectra were recorded using a Waters mass spectrometer. High resolution mass spectra (HRMS) were recorded using an Applied Bio-Sciences HRMS spectrometer at the National Center for Mass Spectroscopy-IICT.
4) on Avance 300 or Avance 500 spectrometers. Chemical shifts (δ) are reported in parts per million (ppm) relative to residual chloroform (CHCl3) (1H: δ 7.26 ppm, 13C: δ 77.00 ppm) as an internal reference. Coupling constants (J) are reported in Hertz (Hz). Peak multiplicity is indicated as follows: s-singlet, d-doublet, t-triplet, q-quartet, m-multiplet and dd-doublet of doublet. All the signals in 13C NMR spectra appeared as singlets and we have performed 13C NMR using CPD (composite pulse decoupling) method. Melting points were measured on a BUCHI melting point machine. IR spectra were recorded on a Thermo Nicolet FT/IR-5700 spectrometer. Mass spectra were recorded using a Waters mass spectrometer. High resolution mass spectra (HRMS) were recorded using an Applied Bio-Sciences HRMS spectrometer at the National Center for Mass Spectroscopy-IICT.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O]H+.
H2O]H+..
; (b) A. Ciegler, A. W. Hayes and R. F. Vesonder, Appl. Environ. Microbiol., 1980, 39, 285–287 CAS
.
.
.
.
; (b) C. S. Reddy, R. V. Reddy, P. K. Chan and A. W. Hayes, J. Environ. Pathol. Toxicol., 1980, 4, 31–37 CAS
.
; (b) K. Mayura, A. W. Hayes and W. O. Berndt, Toxicology, 1982, 25, 311–322 CrossRef CAS
.
; (b) O. E. Sepelgy, S. Haseloff, S. K. Alamsetti and C. Schneider, Angew. Chem., Int. Ed., 2014, 53, 7923–7927 CrossRef PubMed
; (c) K. C. Nicolaou, H. Yu and M. Wartmann, Angew. Chem., Int. Ed., 2005, 117, 766–771 CrossRef PubMed
; (d) E. J. Tisdale, I. Slobodov and E. A. Theodorakis, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12030–12035 CrossRef CAS PubMed
; (e) B. Franck, J. Stöckigt, U. Zeidler and G. Franckowiak, Chem. Ber., 1973, 106, 1198–1220 CrossRef CAS PubMed
.
.
.
.
.
.
.
.
.
.
.
.
.
; (b) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed
; (c) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed., 1993, 32, 131–163 CrossRef PubMed
; (d) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed., 1993, 105, 137–170 CrossRef CAS PubMed
.
; (b) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 118, 7292–7344 CrossRef PubMed
.
; (b) G. L. Zhao and M. Shi, Tetrahedron, 2005, 61, 7277–7288 CrossRef CAS PubMed
.
; (b) W. Liu and G. Zhao, Org. Biomol. Chem., 2014, 12, 832–835 RSC
; (c) W. H. Miles, P. G. Gildner, Z. Ahmed and E. M. Cohen, Synthesis, 2010, 23, 3977–3979 CrossRef
.
; (b) L. C. Rao, H. M. Meshram, N. S. Kumar, N. N. Rao and N. J. Babu, Tetrahedron Lett., 2014, 55, 1127–1131 CrossRef PubMed
; (c) L. C. Rao, N. S. Kumar, V. Dileepkumar, U. S. N. Murthy and H. M. Meshram, RSC Adv., 2015, 5, 28958–28964 RSC
; (d) L. C. Rao, N. S. Kumar and H. M. Meshram, Helv. Chim. Acta, 2015, 98, 978–985 CrossRef CAS PubMed
; (e) L. C. Rao, N. S. Kumar, N. J. Babu and H. M. Meshram, New J. Chem., 2015 10.1039/c5nj01377a
.















