Evaluation of the efficiency of brackish desalination ion exchange membranes using electrodialysis process†
Krishnaveni Venugopal and
Sangeetha Dharmalingam*
Department of Mechanical Engineering, Anna University, Guindy, Chennai 600025, Tamil Nadu, India. E-mail: sangeetha@annauniv.edu; Fax: +91 044 22357744; Tel: +91 044 22357763
First published on 24th August 2015
Abstract
In this study, polysulfone (PSu) was functionalized and modified using resin and fiber reinforcements for the preparation of mono and bipolar ion exchange membranes. In the case of the bipolar membrane, platinum was used as the intermediate layer. A solution of NaCl in the brackish water concentration range from 5 g L−1 to 25 g L−1 was used as the feed solution for the desalting technique using an electrodialysis process. A commercially procured ion exchange membrane made of polystyrene divinyl benzene was also evaluated for the purpose of comparison. The BPMED performance reached a highest current efficiency of 82.5% and 53.6% with the energy consumption of 0.52 W h and 1.39 W h for the synthesized and commercial membranes respectively. Also the performance of the fabricated unit was assessed in terms of electrical conductivity, salinity and ion (sodium and chloride) concentrations for the feed solution after an 8 hour duration. The reproducibility performance of the membranes was also analyzed for the synthetic salt solution.
1 Introduction
To satisfy the demand for a more reliable high quality water supply and other basic needs because of the increased population and pressure for both consumptive and non-consumptive uses, desalination techniques are a focus of all countries. But a desalination technique alone cannot deliver the promised amount of improved water supply. The ability to make the best use of desalination is a subject at present for a number of wider water-related researchers. The most common desalination processes used are distillation and membrane processes. Historically, though distillation technologies have dominated the seawater desalination market, due to its disadvantages the most new desalination plants use membrane technologies instead of the distillation technique. The quality of the feed water is one of the determining factors in deciding on the type of membrane process to use.1 In membrane based desalination technology the utilized electric energy consumption is almost the same or lower than for distillation and it does not need any thermal energy.The new membrane based electrodialysis (ED) process was commonly used for the production of quality water through the desalination technique. The energy consumption utilized for the process is directly related to the salinity of the feed water. Membrane based ED processes have considerable advantages in desalting brackish water and are used over a wide range of salinity concentration levels from brackish to sea water.2 Many types of membranes exist due to classification under various criteria. Generally ion exchange membranes (IEMs) can be defined as a thin layer of electrolytic barrier which can separate two phases and restrict the transport of various chemical species through it; they are subdivided into cation exchange membranes (CEMs), anion exchange membranes (AEMs) and bipolar membranes (BPMs).3
Monopolar membranes (CEM & AEM) were prepared by functionalization methodologies whereas BPM can be prepared by simply laminating conventional CEM and AEM.4 But laminated BPMs prepared with anion and cation exchange layers (CEL/AEL) alone, often do not perform efficient water dissociation.5 But the presence of heterogeneous materials like ion-exchange resin (IER) particles in non-conducting polymer matrix layers of a BPM and the presence of catalytic intermediates like quaternary6 and non-quaternary amine groups,7 weak acids such as phenolic, carboxylic acid or phosphoric acid groups8 and their corresponding base, inorganic substances such as Mg, Ni, Co, Mn, Cu, Fe and Al,9 metallic compounds such as ruthenium tri-chloride, chromic nitrate, indium sulfate, hydrated zirconium oxide, etc.,10,11 heavy/noble metal ions like Cr3+, Fe2+, Ag, Au, Pt, Pd, Os, Rh, Ti, Sn, Zr, Pa, Ru etc.,12,13 macromolecules such as polyethylene glycol (PEG),14 polyvinyl alcohol (PVA)15 and bovine serum albumin (BSA) containing both carboxylic and amino groups,16 starburst dendrimer polyamidoamine (PAMAM)17 etc.,4,5 in between the CEL and AEL as an intermediate layer (IL), usually results in a BPM with higher mechanical stability18,19 with improved water dissociation effects (the rate was found to be 50 million times faster than the ordinary water dissociation in the presence of a catalyst). Hence in the present study, all the IEMs were prepared with resin and glass fiber reinforcements.
Managing high salinity concentrates generated during membrane water desalination processes was a primary need to be addressed due to its potential threat to the receiving ecosystems regarding brine discharge. Depending on the desired application, bipolar membrane electrodialysis (BPMED) processes can be performed with different stack configurations. Tongwen & Weihua20 evaluated the effect of cell configurations such as A–C (type I), C–BP–C (type II) and BP–A–C–BP (type III) on the production of citric acid using BPMED. From the results, magnitudes of cell voltage, CE and energy consumption followed the analogous order as type II < type I < type III, while the potential drop across a BPM followed the order type II > type I ≈ type III suggesting that type II seemed to be a favorable cell configuration for the production of citric acid. Nagasawa et al.21 used both conventional ED and five compartmental BPMED techniques separately to remove boron from sodium tetra borate solution containing 100 mg L−1 of boron. The results confirmed that with the use of BPMED, more than 90% of the boron was removed under both acidic (pH 2.3) and basic (pH 9.1) conditions, whereas only 35–45% of the boron was removed using conventional ED. Nataraj et al. studied the ED pilot plant unit coupled with a membrane stack containing 11 cation-exchange and 10 anion-exchange membranes for the removal of nitrates and hardness from simulated aqueous mixtures containing salts that are usually encountered in brackish water. More than 94% of the nitrates, 89% of the chlorides, and 86% of the TDS were successfully removed after 150 min of operation of the ED.22 Using the same membrane stack configuration, wastewater effluent from the paper industry could be treated. The obtained results show that 80% of wastewater could be recovered by the use of the MF/ED hybrid process, while the remaining 20% of flow (concentrate) can be used as a biomass.23
The present study highlights the work designed to evaluate the desalination efficiency of prepared monopolar (CEM and AEM) and bipolar (with platinum as IL) IEM with resin and glass fiber reinforcements using polysulfone (PSu). The use of platinum has been given the greatest economic importance in various applied fields such as automobiles, oil refining, glass-making, medical instruments, electronics, dentistry, jewellery, computers, fertilizers, plastics, fuel cells, etc. which has also urged its use in water treatment applications. In addition, its certain properties such as superior conductivity, resistance to oxidation and strong catalytic property compared to other types of intermediates have shown platinum to be one of the best choices to be used as an intermediate for BPM in the present study. The study was conducted for five different synthetic salt water concentrations ranging from 5 g L−1 to 25 g L−1 of NaCl solution (used in feed compartment (FC)) in terms of conductivity, solution pH, transport number of ions (T. No.), feed concentration, current efficiency (CE), energy consumption, water dissociation efficiency (WDE), water dissociation flux and acid–alkali production in the acid compartment (AC) and base compartment (BC) up to 8 h. The stack performance using the synthesized membranes was compared with that of the commercial polystyrene divinyl benzene based (PSDVB) IEM under similar experimental conditions. In addition, the decrease in sodium-chloride ion concentration, salinity and electrical conductivity of the feed water were observed and the reproducibility test for the membrane system with the highest CE was conducted.
2 Materials and methods
Commercial strong acid cation exchange membranes (CMI – 7000S) and commercial strong base anion exchange membranes (AMI – 7001S) were procured from Membranes International INC, New Jersey, USA. BPMs made up of PSDVB were procured from Arun Electro chemicals, Chennai. Glass fiber was purchased from Meena glass fiber industry. Seralite (cation exchange resin (CER) – equivalent to Amberlite IRC-120, 20–50 mesh standard grade) and Seralite (anionic exchange resin (AER) – equivalent to Amberlite IRA-400, 20–50 mesh standard grade) were obtained from Sisco Research Laboratory Pvt. Ltd (SRL). Platinum chloride (Pt) and polysulfone (PSu) [Mn = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (MO), Mw = 35
000 (MO), Mw = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (LS)] were purchased from Aldrich (USA).
000 (LS)] were purchased from Aldrich (USA).
2.1 Reinforced IEM preparation
Anionic and cationic functionalized ionomer membranes of sulfonated polysulfone (SPSu) and quaternized polysulfone (QPSu) were prepared as per the procedure reported earlier.24 To introduce additional functions like higher ion exchange capacity (IEC) with firmness and durability for a polymer separation membrane, its surface was modified.25 Reinforced cationic exchange membrane (RCEM) and reinforced anionic exchange membrane (RAEM) based on PSu polymer were prepared by first dispersing a specific quantity26 (from 10% to 70%) of dried (60 °C for 24 h in an oven) and crushed cationic/anionic exchange resin (CER/AER) in either SPSu–N,N′-dimethylformamide (DMF) or QPSu–DMF solution, respectively, for 12 h using a magnetic stirrer at room temperature. In order to break the aggregates and to obtain a uniform dispersion, the solution was sonicated for 30 min. Then, the solution was cast on a clean glass petridish and the glass fiber matrix was placed to be immersed in the solution before drying in the oven for 24 hours at 80 °C. The IEC value for the prepared SPSu and QPSu were found to be 1.47 and 1.57 meq g−1, respectively. Also, as expected, the reinforced synthesized monopolar membrane had a higher IEC value of 4.75 meq g−1 for RSPSu and 4.35 meq g−1 for RQPSu, respectively.The obtained membranes with various resin content were subjected to conductivity studies as described in the ESI S1†; from Table 1it is observed that the conductivity of the prepared membranes increased with the increase in IER loadings. It was optimized at 40% for both resins26 because beyond 50%, the nature of the membranes changed to brittle from their soft nature. Reinforced bipolar membrane (RBPM i.e. RPSu–Pt) was prepared using RCEM (RSPSu) and RAEM (RQPSu) as CEL and AEL, respectively. Then on one side of both the CEL and AEL layers, a solution containing approximately 6 mL of 0.12 g of Pt-containing solution was coated on to form the IL. Finally the IL-coated sides of both layers (CEL and AEL) were sandwiched and subjected to a hot press to finally obtain RBPM represented as RBPM–Pt as shown in Fig. 1.
| % of resin added | CER conductivity (S cm−1) | AER conductivity (S cm−1) |
|---|---|---|
| 10 | 1.68 × 10−6 | 8.69 × 10−7 |
| 20 | 2.78 × 10−6 | 4.78 × 10−6 |
| 30 | 5.31 × 10−6 | 7.57 × 10−6 |
| 40 | 4.76 × 10−5 | 3.8 × 10−5 |
| 50 | 3 × 10−5 | 2.73 × 10−5 |
| 60 | 2.39 × 10−6 | 1.79 × 10−6 |
| 70 | 1.7 × 10−6 | 1.2 × 10−6 |
2.2 Design and working principle of the BPMED unit
The BPMED unit used in the study for the determination of the desalination process efficiency was supplied by Arun Electrochemical, Chennai.27 The BPMED unit consisted of a DC power supply and membrane ED stack. The construction and description of the used BPMED unit is shown in Fig. 2. The cathode and anode electrodes used were stainless steel and Ti coated with Ti–Ru–Pd oxides, respectively. The active membrane area of each membrane and volume of each chamber was about 120 cm2 and 160 cm3, respectively. Each compartment was connected to a tank with a capacity of 1 L, allowing for batch mode recirculation of external solutions by submersible pumps. The experiments were carried out at the initial voltage of 10 V and the same electric field was applied for the solutions with different ranges of feed concentration. In order to minimize the cell voltage generated during the initial stages of the performance, dilute HCl (0.01 N) and dilute NaOH (0.01 N) solutions were used in AC and BC, respectively. 0.05 mol L−1 of NaCl solution was used in each EC. Because at lower concentrations, higher resistances between membranes resulted and at higher concentrations, the selectivity nature of the IEMs became lower, while NaCl solutions of concentrations ranging from 5 g L−1 to 25 g L−1 were taken in the FC in order to avoid a higher rate of salt ion leakage across the BPM and formation of undesired salt impurities in products during BPMED performance. During the performance, at every 15 min time interval, various process parameters were evaluated using the same set of equations as reported earlier.27 After 8 h of treatment the final solutions of various synthetic feed concentrations and reproducibility test samples were analyzed for their sodium chloride ion concentration, salinity and electrical conductivity.3 Results and discussion
3.1 pH change in various compartments with time
Fig. 3 and 4 represent the variation of solution pH in FC, EC, AC and BC over time for the brackish water feed concentration range including its reproducibility for both laboratory synthesized RPSu–Pt IEM and commercially procured PSDVB IEM systems. It was clear from these figures that with the increase in salt or feed concentration, the initial pH value of the corresponding solution was also found to be higher in FC for both IEM systems. In the case of the RPSu–Pt IEM system (Fig. 3), the pH in FC showed an acidic nature at the final stages after an increase in its pH towards basicity at the initial stages of the performance. This is because with increasing time, higher quantities of acid were produced due to water dissociated products thereby resulting in proton leakage through IEMs depending on the capacity of protons to undergo back diffusion.28 On the other hand, as per Fig. 4 in the case of the PSDVB IEM system, the feed solution finally became basic in nature for all feed concentrations. The PSDVB based cell experienced a greater leakage of ions from BC to FC and thus it remained basic in nature. Moreover, proton leakage through IEM was low due to the low acid concentration that was produced during the BPMED process using PSDVB IEM systems.29 | ||
| Fig. 3 pH changes in FC, EC, AC and BC with time for various feed concentrations of the RPSu–Pt IEM system. | ||
 | ||
| Fig. 4 pH changes in FC, EC, AC and BC with time for various feed concentrations of the PSDVB IEM system. | ||
Whereas, in the case of EC, since 0.05 M NaCl was taken as the electrolyte solution each time, the initial pH remained constant for all performances. Though both systems showed the final solution to be acidic in nature, the acidity was greater for the PSDVB IEM system than for the RPSu–Pt IEM system. The difference in pH observed between the two types of IEM systems in FC and EC was mainly attributed to the leakage of ions occurring through membranes arranged between the compartments in a stack. Similarly in the case of AC and BC, the solution pH was found to be approximately around 2.45 and 10.74, respectively, for all performances because of the dilute acid and base solutions that were taken initially.
From Fig. 3 and 4, it was additionally observed that, irrespective of whether it was AC or BC, the acidity or basicity was found to be increased during the first half stage of a performance and the increase was not uniform because of the leakage of some ions into the neighboring compartments. The nature of pH change reflected their acid and base production for both PSDVB and RPSu–Pt IEM systems. This pH change in various compartments clearly suggested that both IEM systems possessed adequate capacity to split water into its co-ions under the influence of an electric field. Also, it is observed that for the reproducibility test, more or less the same result as that of its original feed concentration was noticed.
3.2 Variation of acid, base and feed conductivities with time
The changes in pH value are justified using the respective conductivity values. Since the ionic mobility of protons was higher than that of the hydroxyl ions, correspondingly, the conductivity value was found to be higher in the case of AC when compared with BC. For the RPSu–Pt IEM system, from Fig. 5, the highest acid and base conductivity observed was 1.31 mS cm−1 and 0.84 mS cm−1, respectively. After reaching the highest conductivity, either a decrease or an increase was expected mainly due to the loss of ions from acid–base compartments or the introduction of other ions from the neighboring compartments. In the case of FC, a wide range in the decrease of conductivity30,31 was observed which was then followed by an increase that did not exceed the initial conductivity of that particular feed concentration. | ||
| Fig. 5 Conductivity changes in AC, BC and FC for different feed concentrations with time for the RPSu–Pt IEM system. | ||
For the PSDVB IEM system, from Fig. 6, the highest acid and base conductivity values were found to be 1.29 mS cm−1 and 0.7 mS cm−1, respectively. In addition, the lower acid and base conductivity values for 5 g L−1 among other feed concentrations indirectly proved its lower acid and base pH change. In the case of FC the conductivity initially decreased depending upon the feed concentration, which then increased slightly and finally remained constant over time. This increased conductivity was observed to be a little higher than its initial value of that particular feed concentration mainly because of the leakage of ions that occurred from the neighboring compartments through the IEM into FC. It should also be noted that the reproducibility test in both these cases showed more or less the same results as that of its original feed concentration.
 | ||
| Fig. 6 Conductivity changes in AC, BC and FC for different feed concentrations with time for the PSDVB IEM system. | ||
3.3 Effect of the BPMED process on acid–base production
When the entire BPMED cell was kept under an electric field using electrodes, due to the large electric field appearing at the membrane interface, an excess of OH− and H+ ions was produced due to the field enhanced chemical reaction as per the Second Wien effect. Along with this, Na+ and Cl− ions were also continuously transported through IEMs from FC into BC and AC, respectively, resulting in the formation of acid and base of certain concentrations32 which was evidently proved by pH and conductivity studies. The maximum concentration of both NaOH and HCl depended on feed concentration, time, IEC and the functional group nature of that membrane.However, Fig. 7(a) clearly shows that the RPSu–Pt IEM system produced higher acid and base concentrations of about 0.014 N and 0.006 N for the same feed concentration. It has to be noted that once the higher concentration of acid/base was reached in a particular feed concentration, it remained constant until a certain duration of time after which it decreased with the increase in process time due to a decrease in NaCl concentration or diminished mass transfer of Na+ and Cl− ions in the feed solution.
 | ||
| Fig. 7 Changes in acid–base yield with time for various feed concentrations for (a) the RPSu–Pt IEM system and (b) the PSDVB IEM system. | ||
It was observed from Fig. 7(b) that the PSDVB IEM system showed higher acid and base concentrations of about 0.009 N and 0.006 N respectively for various feed concentrations. Though the PSDVB based IEM cell is meant for base production rather than acid production because of higher specific permselectivity of CEM for H+ ions as reported in the literature,26 in the present study, from Fig. 7(b), it was observed that the alkalinity concentration was lower in the case of the PSDVB IEM system. Furthermore, H+ ions in the presence of water have a higher intrinsic mobility than OH− ions thereby resulting in more leakage of H+ ions through the AEM which leads to a decrease in the concentration of acid in AC.27 A similar decreasing trend was also observed in the case of BC for various feed concentrations.
In addition it should be noted that irrespective of the type of system used (either RPSu–Pt IEM or PSDVB IEM system), the produced acid and base values purely represent the acid and base generated during the process because the concentration value obtained from the titration procedure was subtracted from its initial concentration value (0.01 N). Thus the final value is solely due to the produced acid and base. Again, it should be noticed that the reproducibility test in both types of IEM systems showed more or less the same results as that of its original feed concentration.
3.4 Ion transport number and WDE changes with time
Since CE depends upon the ion transport and ionic mobility, for a better process efficiency it was expected that the system should have both a higher T. No. of ion and better WDE. Fig. 8 and 9 represent the T. No. properties of both sodium and chloride ions and the WDE for the RPSu–Pt and PSDVB IEM systems. From these figures it was clear that depending upon the membrane capacity, time and feed concentration, the T. No. of both Na+ ion and Cl− ions was observed to have a higher value at its initial stage and then started decreasing with time for both types of IEM system. In the case of WDE, both systems showed the lowest value initially which then increased with time and then remained constant. This is because at the beginning of the BPMED process, since the NaCl concentration was higher in FC, a transfer of a large amount of Na+ and Cl− ions through the IEM was observed. But with increasing time due to the decrease in NaCl concentration, the current was carried by water dissociated products such as H+ and OH− ions.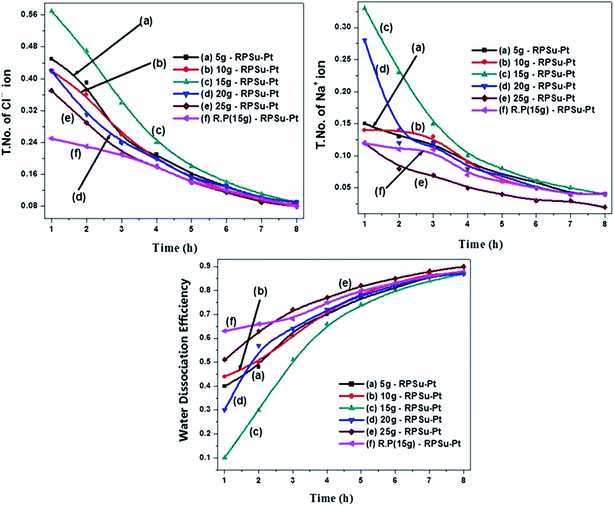 | ||
| Fig. 8 Changes in the T. No. of ions and WDE for various feed concentrations with time for the RPSu–Pt IEM system. | ||
 | ||
| Fig. 9 Changes in the T. No. of ions and WDE for various feed concentrations with time for the PSDVB IEM system. | ||
From Fig. 8, it can be observed that the RPSu–Pt IEM system showed the highest T. No. of 0.57 and 0.33 for chloride and sodium ions, respectively, for a 15 g L−1 feed concentration. Similarly from Fig. 9, it is observed that the PSDVB IEM system showed a greater T. No. of 0.16 and 0.08 of chloride and sodium ions respectively, for the same feed concentration.33 While in the case of WDE, as per Fig. 8, the RPSu–Pt IEM system showed results of about 0.87 finally for different feed concentrations. The reason for this difference was mainly due to the salt concentration in FC and hence, when the feed concentration was lower it was a better opportunity for the transport of water dissociated ions. But when the feed concentration was higher, the competition between these ions would be higher and more preferably the salt ion transport occurred initially followed by the water dissociated ions.34 Whereas, as per Fig. 9, the highest WDE value observed for the PSDVB IEM system was about 0.07. The highest T. No. and steady increase in WDE with time observed for the RPSu–Pt IEM system was mainly due to the increase in electric field, pre-polarization of water molecules at the membrane–solution interface and the presence of a catalytic Pt intermediate in between the two monopolar layers of the BPM.35 In the reproducibility test for the RPSu–Pt IEM systems, a little lesser value was obtained for T. No. of both ions whereas WDE showed a little higher performance when compared with that of its original feed concentration performance. For the PSDVB IEM system, the T. No. of both chloride and sodium ions was observed to be little lesser and the WDE showed a more or less same performance.
3.5 Determination of process efficiency parameters with time
For any system, a higher CE with a lower energy consumption is one of the factors which determine the feasibility of any electrochemical process towards higher process efficiency. Fig. 10 represents the variation of CE and energy consumption of both RPSu–Pt and PSDVB IEM systems with time for various feed concentrations. It was observed that both CE and energy consumption increased with the increase in feed concentration due to the same reason as discussed for T. No. of ions and WDE. With the increase in time, CE was observed to decrease for each feed concentration mainly due to leaching out of resin particles from the functionalized polymer because of ballooning, and the flexible and higher elongation nature of the polymer when placed in water for a longer duration of time. The reason for the increase in energy consumption with time was mainly due to the electrical resistance in various compartments. The increase of resistance in FC resulting from the exhaustion of NaCl in the solution can be offset by the decrease in electrical resistance in AC and BC caused by the increase of acid–base concentrations as a consequence of transfer of Cl− and Na+ ions from the feed solution.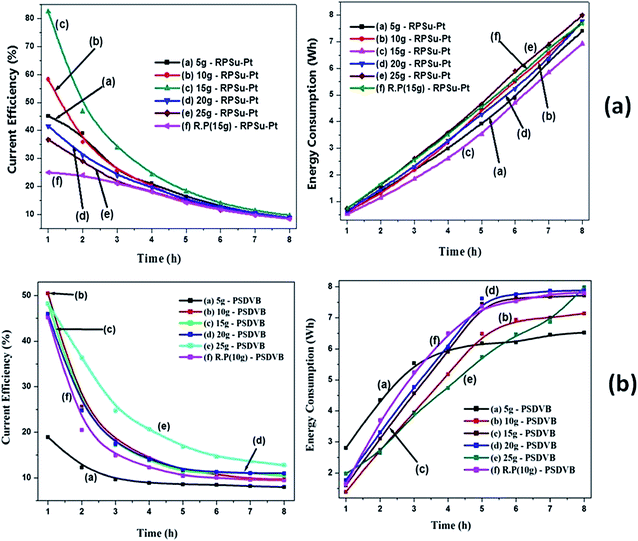 | ||
| Fig. 10 Variation of CE and energy consumption for various feed concentrations with time for (a) the RPSu–Pt IEM system and (b) the PSDVB IEM system. | ||
In the case of the RPSu–Pt IEM system, as per Fig. 10(a), the highest CE and energy consumption values observed were about 82.5% and 0.52 W h respectively, for a 15 g L−1 feed concentration. In the case of the PSDVB IEM system the same was observed to be 53% and 1.39 W h respectively, for a 10 g L−1 feed concentration, as shown in Fig. 10(b). From Fig. 10(a), it can be observed that the RPSu–Pt IEM system showed a uniform increase in energy consumption with time until the final stages of experiment without any observation of oscillations with respect to various feed concentrations. This was mainly because of the presence of IER in addition to the fiber reinforcements during the membrane preparation which created an increased electrical resistance. So to overcome this additional resistance, the RPSu–Pt IEM system consumed a little additional energy till the final stage of a performance resulting in the steady increase in value. Whereas from Fig. 10(b), it can be seen that, though PSDVB IEM system displayed oscillations at the initial stages, its increase in energy consumption with time finally depended upon its feed concentration. In terms of the reproducibility test, the CE was observed to be lower and the energy consumption was observed to be higher than its original feed concentration for both types of IEM system because the membrane loses some of its IEC and functional groups depending on its usage time.
3.6 Variation of current, potential and water dissociation fluxes with time
Fig. 11 and 12 reveal the relationship between current, potential and water dissociation flux of protons and hydroxyl ions with time for various feed concentrations of both RPSu–Pt and PSDVB IEM systems. Similar to the results of Michael Rajesh et al.35, from Fig. 11 and 12, it was clear that the current increased with time for both systems depending upon the feed concentration. The reason was mainly because of the production of OH−/H+ during water dissociation and the overall decrease in stack resistance due to the resistance difference in various compartments caused by IEM. The maximum current observed finally for the lowest and highest feed concentrations was about 86 mA and 89 mA, respectively, for RPSu–Pt and it was about 105 mA and 89 mA, respectively, for the PSDVB IEM system. | ||
| Fig. 11 Variation of current, potential, protons and hydroxyl ion water dissociation fluxes with time for the RPSu–Pt IEM system. | ||
 | ||
| Fig. 12 Variation of current, potential, protons and hydroxyl ion water dissociation fluxes with time for the PSDVB IEM system. | ||
The voltage variation slightly increased with time and attained a maximum value, depending upon feed concentration which then decreased. This is because the production of acid and alkali increased with the increase in voltage to some extent, after which further increase in voltage deteriorated the membrane properties as discussed by Trivedi et al.36 The highest voltage reached by the RPSu–Pt IEM system among the various feed concentrations was about 11.5 V, as per Fig. 11, and it was about 23.1 V for the PSDVB IEM system, as can be seen from Fig. 12. The reason for the observed lower voltage for the RPSu–Pt IEM system can be explained theoretically using a protonation and de-protonation reactions model and the hydrophilicity change in the interface.14
From Fig. 11 and 12, it was clear that for both IEM systems, whether acidic or basic, the water dissociation flux was observed to decrease with time for the various feed concentrations mainly due to the depletion of Na+ ions in the anode compartment and Cl− ions in the cathode compartment. As per Fig. 11, the higher acid and basic fluxes observed were about 37 × 10−6 mol m−2 s−1 and 14.8 × 10−6 mol m−2 s−1 in AC and BC respectively, for the RPSu–Pt IEM system. And for the PSDVB IEM system the same was about 14.8 × 10−6 mol m−2 s−1 and 3.7 × 10−6 mol m−2 s−1, respectively. The greater flux values for the RPSu–Pt IEM system confirmed the catalytic activity of the Pt intermediate layer at the BPM interface by means of hydrogen bonding and polar interaction between Pt and water molecules.
In the reproducibility test in the case of the RPSu–Pt IEM system, both acidic and basic fluxes were observed to be lower than its original feed concentration. Whereas the current change was a higher value and the potential change was also higher at the initial time and then fluctuated around its original feed concentration during the later stage of the performance. In the case of the PSDVB IEM system, the reproducibility test revealed that the dissociation fluxes were lower than the original feed concentration, while both potential and current values were observed to be higher.
3.7 Determination of electrical conductivity, salinity and sodium-chloride ion concentration
Tables 2 and 3 give the electrical conductivity, salinity and sodium-chloride ion concentrations in 100 mL of various feed sample solutions for both RPSu–Pt and PSDVB IEM systems. It should be noted that all these parameters increased with the increase in feed concentration mainly because of two reasons: (i) increased NaCl concentration in the feed solution and (ii) restricted transport of sodium and chloride ions through IEMs because of decreased membrane capacity due to the adhesion of the salt ions on the surface of the membrane, especially with higher feed concentrations and at a longer duration of time. Though the transfer of Na+ and Cl− ions from FC to neighboring compartments under the electric field was confirmed by the lower electrical conductivity and sodium and chloride ion concentrations of final feed solutions, the effectiveness of the process for both types of IEM systems was confirmed through salinity measurements (Tables 2 and 3). The higher difference between the initial and final values for all these parameters represents the process effectiveness in removal of NaCl and higher acid–base production. The reproducibility test results can also be discussed in a similar way.| Final feed sample solution | Electrical conductivity (mS cm−1) | Salinity (%) | Chloride ion concentration (mg per 100 mL) | Sodium ion concentration (ppm) | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| 5 g L−1 | 12.53 | 6.84 | 8.2 | 4.2 | 35.96 | 17.55 | 42.9 | 11.7 |
| 10 g L−1 | 20.90 | 8.2 | 14.3 | 5.2 | 39.12 | 22.5 | 44.4 | 15.5 |
| 15 g L−1 | 30.40 | 10.69 | 21.3 | 7.0 | 46.69 | 26.1 | 46.2 | 17.4 |
| 20 g L−1 | 37.7 | 12.55 | 26.9 | 8.3 | 54.26 | 26.4 | 48.0 | 17.6 |
| 25 g L−1 | 44.9 | 15.34 | 33.1 | 10.2 | 58.68 | 31.95 | 49.1 | 21.3 |
| Reproducibility test (15 g L−1) | 30.40 | 10.68 | 21.3 | 7.1 | 46.69 | 24.3 | 46.2 | 16.2 |
| Final feed sample solution | Electrical conductivity (mS cm−1) | Salinity (%) | Chloride ion concentration (mg per 100 mL) | Sodium ion concentration (ppm) | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| 5 g L−1 | 12.53 | 11.30 | 8.2 | 7.3 | 35.96 | 20.8 | 42.9 | 24.5 |
| 10 g L−1 | 20.90 | 19.4 | 14.3 | 13.9 | 39.12 | 27.3 | 44.4 | 38.9 |
| 15 g L−1 | 30.40 | 27.5 | 21.3 | 19.1 | 46.69 | 30.4 | 46.2 | 44.3 |
| 20 g L−1 | 37.7 | 31.3 | 26.9 | 22.1 | 54.26 | 37.6 | 48.0 | 45.3 |
| 25 g L−1 | 44.9 | 36.9 | 33.1 | 26.6 | 58.68 | 43.4 | 49.1 | 46.5 |
| Reproducibility test (10 g L−1) | 20.90 | 20.4 | 14.3 | 14.0 | 39.12 | 28.7 | 44.4 | 40.0 |
4 Conclusion
Functionalized PSu based resin-glass fiber reinforced monopolar and bipolar (with Pt intermediate) IEMs were prepared and compared with commercial PSDVB IEM in order to evaluate their desalination efficiency for various feed concentrations using BPMED technology. Based on the results obtained for various process parameters such as current efficiency (82.5% for RPSu–Pt and 53.61% for PSDVB), energy consumption (0.52 W h for RPSu–Pt and 1.39 W h for PSDVB), acid–base production (0.014 N acid & 0.006 N base for RPSu–Pt and 0.009 N acid & 0.006 N base for PSDVB) and WDE (0.87 for RPSu–Pt and 0.07 for PSDVB), it can be concluded that the RPSu–Pt IEM system showed a better performance than the commercial PSDVB IEM system. Also the electrical conductivity, salinity and sodium-chloride ion concentration results were observed to be better for the RPSu–Pt IEM system than for the PSDVB IEM system due to the presence of a catalytic intermediate region in the RPSu–Pt based IEM system.Acknowledgements
Financial support from the Board of Research in Nuclear Science (BRNS), Mumbai, India (Letter No. 2010/37C/1/BRNS/826, Dated: 28-06-2010) is gratefully acknowledged.References
- V. A. Shaposhnik, V. I. Vasileva, R. B. Ugryumov and M. S. Kozhevnikov, Russ. J. Electrochem., 2006, 42, 531–537 CrossRef CAS.
- T. Younos and K. E. Tulou, Journal of Contemporary Water Research & Education, 2005, 132, 3–10 Search PubMed.
- H. Strathmann, Electrodialysis and related processes, in Membrane Separation Technology – Principles and Applications, ed. R. D. Nobe and S. A. Stern, Elesevier Science B.V., 1995 Search PubMed.
- V. J. Frilette, J. Phys. Chem., 1956, 60, 435–439 CrossRef CAS.
- A. Tanioka, K. Shimizu, K. Miyasaka, H. J. Zimmer and N. Minoura, Polymer, 1996, 37, 1883 CrossRef CAS.
- R. Simons, Electrochim. Acta, 1984, 29, 151–158 CrossRef CAS.
- R. Simons, Electrochim. Acta, 1985, 30, 275–282 CrossRef CAS.
- N. V. Sheldeshov, N. P. Gnusin, V. I. Zabolotskii and N. D. Pis’menskaya, Sov. Electrochem., 1987, 22, 742 Search PubMed.
- Y. Tanaka, S. Matsuda, Y. Sato and M. Seno, J. Electrochem. Soc., 1982, 50, 667–672 CAS.
- M. S. Kang, Y. J. Choi, H. J. Lee and S. H. Moon, J. Colloid Interface Sci., 2004, 273, 523–532 CrossRef CAS PubMed.
- M. S. Kang, Y. J. Choi and S. H. Moon, J. Colloid Interface Sci., 2004, 273, 533–539 CrossRef CAS.
- F. Hanada, K. Hirayama, S. Tanaka and N. Ohmura, Bipolar membrane and method for its production, JP Patent 2524012, 1996.
- F. G. Wilhelm, N. F. A. van der Vegt, M. Wessling and H. Strathmann, Bipolar membrane preparation, in Bipolar membrane Handbook, ed. A. J. B. Kemperman, Twente University Press, Enschede, The Netherlands, 2000, pp. 79–108 Search PubMed.
- R. Q. Fu, T. W. Xu, G. Wang, W. H. Yang and Z. X. Pan, J. Colloid Interface Sci., 2003, 263, 386–390 CrossRef CAS.
- R. Q. Fu, Y. H. Xue, T. W. Xu and W. H. Yang, J. Colloid Interface Sci., 2005, 285, 281–287 CrossRef CAS PubMed.
- R. Q. Fu, T. W. Xu, W. H. Yang and Z. X. Pan, J. Colloid Interface Sci., 2004, 278, 318–324 CrossRef CAS PubMed.
- R. Q. Fu, T. W. Xu, Y. Y. Cheng, W. H. Yang and Z. X. Pan, J. Membr. Sci., 2004, 240, 141–147 CrossRef CAS PubMed.
- B. Bauer, F. J. Gerner and H. Strathmann, Desalination, 1988, 68, 279 CrossRef CAS.
- O. Kedem and A. Warshawsky, A supported, mechanically stable bipolar membrane for electrodialysis, EP 0,504,904 A2, Yeda Research and Development Company, Ltd., Israel, 1992.
- X. Tongwen and Y. Weihua, J. Membr. Sci., 2002, 203, 145–153 CrossRef.
- H. Nagasawa, A. Iizuka, A. Yamasaki and Y. Yanagisawa, Ind. Eng. Chem. Res., 2011, 50, 6325–6330 CrossRef CAS.
- S. K. Nataraj, K. M. Hosamani and T. M. Aminabhavi, J. Appl. Polym. Sci., 2006, 99, 1788–1794 CrossRef CAS.
- S. K. Nataraj, S. Sridhar, I. N. Shaikha, D. S. Reddy and T. M. Aminabhavi, Sep. Purif. Technol., 2007, 57, 185–192 CrossRef CAS PubMed.
- V. Krishnaveni and D. Sangeetha, Desalination, 2012, 296, 37 CrossRef PubMed.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS PubMed.
- M. Kumar and V. K. Shahi, J. Membr. Sci., 2010, 349, 130–137 CrossRef CAS PubMed.
- K. Venugopal and S. Dharmalingam, Desalination, 2014, 344, 189–197 CrossRef CAS PubMed.
- Y. Wang, X. Zhang, C. Huang and T. Xu, J. Membr. Sci., 2011, 385, 226–233 CrossRef PubMed.
- H. Strathmann and G. H. Koop, Process economics of the electrodialytic water dissociation for the production of acid and base, in Handbook on Bipolar Membrane Technology, ed. A. J. B. Kemperman, Twente University Press, Enschede, The Netherlands, 2000, pp. 193–218 Search PubMed.
- S. Shi, Y. H. Lee, S. H. Yun, V. Phan, X. Hung and S. H. Moon, J. Food Eng., 2010, 101, 417–423 CrossRef PubMed.
- M. Badruzzaman, J. Oppenheimer, S. Adham and M. Kumar, J. Membr. Sci., 2009, 326, 392–399 CrossRef CAS PubMed.
- Y. Wang, X. Zhang and T. Xu, J. Membr. Sci., 2010, 365, 294–301 CrossRef CAS PubMed.
- M. Nishapriya and K. Palanivelu, Indian J. Chem. Technol., 2006, 13, 262–268 Search PubMed.
- J. Shen, J. Huang, L. Liu, W. Ye, J. Lin and B. V. Bruggen, J. Hazard. Mater., 2013, 260, 660–667 CrossRef CAS PubMed.
- A. Michael Rajesh, M. Kumar and V. K. Shahi, J. Membr. Sci., 2011, 372, 249–257 CrossRef PubMed.
- G. S. Trivedi, B. G. Shah, S. K. Adhikary, V. K. Indusekhar and R. Rangarajan, React. Funct. Polym., 1996, 28, 243–251 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra10616h |
| This journal is © The Royal Society of Chemistry 2015 |


