Use of porous cellulose microcapsules for water treatment†
Abstract
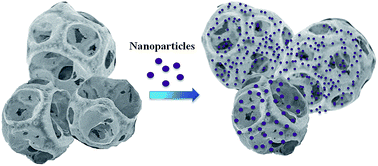
Enhanced usage of metal nanoparticles (NPs) in chemical industry and consumer products is causing undesirable environmental pollution, toxicity, and damage to the ecosystem. New methods are needed to remove such emerging pollutants from the environment. In this paper, we report the synthesis and characterization of polyethylenimine coated porous ethyl cellulose microcapsules and show potential application in the extraction of dissolved citrate or PVP-capped Ag NPs and Au NPs from water. The high surface area of the capsules and affinity of amine functional groups towards metal nanoparticles are used to enhance the extraction efficiency. Kinetics, isotherm models, and pH studies were used to understand the observed extraction efficiency of capsules. The capsules showed interesting Langmuir adsorption capacities of 270 mg g−1 for Ag-citrate, 208 mg g−1 for Ag-PVP, 116 mg g−1 for Au-citrate, and 50 mg g−1 for Au-PVP nanoparticles. We have also developed a simple method to detect metal nanoparticles via modification of the microcapsule with fluorescein isothiocyanate. Overall, our results demonstrate that the porous PEI-crosslinked microcapsules are useful for the extraction of Ag NPs and Au NPs from contaminated water. They can also be modified for the detection of metallic NPs at a ppb level concentration.

 Please wait while we load your content...
Please wait while we load your content...