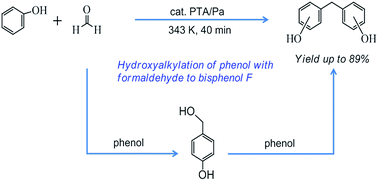
In this study, acid-activated palygorskite (Pa) with tunable surface acidity was obtained by simple acidic treatment of raw clay. The new catalysts with 5–30 wt% 12-phosphotungstic acid (H3O40PW12·xH2O, PTA) were then readily prepared by the wet impregnation method. Their characteristic features were systematically investigated by various means including energy-dispersive X-ray (EDX), X-ray diffraction (XRD), N2 adsorption/desorption isotherms, Fourier-transform infrared spectroscopy (FT-IR), thermo-gravimetric analysis (TGA), as well by comparison with Pa, PTA and H-Beta zeolite. The high activities of these catalysts promoted phenol to undergo hydroxyalkylation, resulting in an interesting bisphenol F (BPF) product. Among these solid acid catalysts, 10% PTA/Pa was chosen as the most suitable catalyst giving an 87% yield and 96% selectivity under mild conditions (phenol/formaldehyde mole ratio of 15 : 1; T = 343 K; catalyst concentration of 0.006 g g−1; 40 min). The surface acid strength and acidic type were characterized by ammonia temperature programmed desorption of NH3 (NH3-TPD), and FT-IR of pyridine adsorption (Py-IR). It was found that the catalytic activity could be further enhanced by impregnating PTA onto Pa due to the enhanced acid strength and the redistribution of Brönsted and Lewis acid sites. Besides, a more appropriate combination of Brönsted and Lewis acid sites was essential to achieve the highest BPF yield. Recycle experiments were conducted and a plausible mechanistic pathway was proposed according to our observations and findings.