Cr(vi) removal by combined redox reactions and adsorption using pectin-stabilized nanoscale zero-valent iron for simulated chromium contaminated water
Abstract
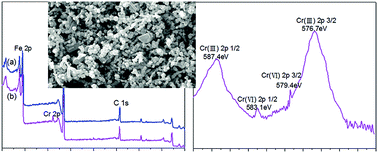
The synthetic pectin-stabilized nanoscale zero-valent iron was used to remove Cr(VI) from simulated chromium contaminated water. The Cr(VI) removal data were well fitted with the pseudo-first order kinetic equation. The observed pseudo-first order rate constant for Cr(VI) removal decreased from 0.0781 to 0.0413 min−1 when the pH increased from 3 to 9. When the initial Cr(VI) concentration increased from 20 to 80 mg L−1, the observed pseudo-first order rate constant decreased from 0.0645 to 0.0366 min−1. The Cr(VI) removal efficiency had obviously increased as the dose of pectin-stabilized nZVI increased from 0.02 to 0.10 g, and it increased from 0.0276 to 0.1159 min−1 as the temperature increased from 15 to 35 °C. The scanning electron microscopy (SEM) images proved that the presence of pectin successfully stabilized the nZVI particles and thus increased the BET specific surface area of nZVI. Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) analyses demonstrated that the mechanisms of Cr(VI) removal by pectin-stabilized nZVI were a combined process of redox reactions and adsorption.

 Please wait while we load your content...
Please wait while we load your content...