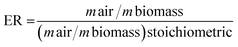Optimization of the microalgae Chlorella vulgaris for syngas production using central composite design
Abdul Raheema,
Wan Azlina W. A. K. G.b,
Y. H. Taufiq Yapb,
Michael K. Danquahc and
Razif Harun*a
aDepartment of Chemical and Environmental Engineering, Universiti Putra Malaysia, 43400 Serdang, Malaysia. E-mail: mh_razif@upm.edu.my; Fax: +6103 8656 7120; Tel: +603 8946 6289
bCatalysis Science and Technology Research Centre, Faculty of Science, Universiti Putra Malaysia, Serdang, Malaysia
cDepartment of Chemical Engineering, Curtin University, Sarawak, Malaysia
First published on 12th August 2015
Abstract
Gasification has emerged as an effective thermochemical conversion technology for generating syngas products from biomass. Process conditions for optimizing the productivity and quality of syngas during gasification vary with the type and composition of the biomass. With escalating research interests in the development of biofuels from microalgae, resulting from its high biomass productivity, agronomical and environmental bioremediation benefits, the current study investigates the optimization of microalgal gasification for syngas production using high temperature horizontal tubular furnace. Four response variables (H2, CO, CO2, and CH4) were optimized under varying conditions of temperature (500–900 °C), microalgal (Chlorella vulgaris) biomass loading (0.6–2.5 g), heating rate (5–25 °C min−1), and equivalent ratio (ER = 0.1–0.35). The optimization study was carried out using central composite design (CCD). Temperature was the most significant process parameter influencing H2 production, followed by microalgal biomass loading and heating rate. An optimum H2 yield of 41.75 mol% was obtained at a temperature of 703 °C, a microalgal biomass loading of 1.45 g, a heating rate of 22 °C min−1, and an ER of 0.29. Statistical analysis showed that the generated models were sufficiently in agreement with the experimental results. It was concluded that the direct gasification of microalgal biomass in the presence of air has significant potential for the commercial-scale production of syngas products.
1. Introduction
Contemporary issues relating to the development of renewable and sustainable fuels are significant as a result of decreasing petroleum fuel reserves and increasing climate change effects. Recent developments in the utilization of biological and agricultural materials have demonstrated a great potential to produce renewable fuels. Amongst these alternative fuel options, syngas from biomass is considered as a promising alternative to fossil fuels for various applications relating to electricity and heat generation, and transportation fuels.1–3 Currently, industrial scale syngas production is predominantly based on fossil fuels, mainly through coal gasification and natural gas reforming.4 However, increasing dependency on fuel and fluctuations in fuel prices have encouraged the production of large volumes of syngas from non-fossil fuel sources for commercial applications. Therefore, biomass such as corn, wheat, grain, sugar cane, and non-food plant biomass has been used as an alternative feedstock to produce syngas.5–8 Notwithstanding, the aforementioned biomass resources may have significant agronomical effects on high value products particularly for food production applications, the requirement for more arable land, technological barriers to product conversion and cost effectiveness. As such, they are less preferable as feedstocks for syngas production.9 Furthermore, these biomass resources contain high levels of lignocellulose, presenting major downstream challenges in the removal or decomposition of residual lignin to enable high syngas conversion and production efficiencies.10,11Microalgal biomass, on the other hand, offers several advantages that support its exploitation as a feedstock for gasification. The absence of lignin, high calorific value, inherent high protein, carbohydrates and lipid content, and low density and viscosity, are some of the key characteristics of microalgal biomass that make it suitable for syngas production.12–14 These advantages endow microalgae with adequate capacities to replace existing syngas production modalities.10,15
Amongst the thermochemical conversion technologies, gasification has proven to be an effective technology for biomass conversion into gaseous products. Gasification is versatile and suitable for a wide spectrum of biomass types such as algae, wood, peat, waste and other organic residues as feedstocks for a wide range of applications.16–18
During gasification, the feedstock is partially oxidized to undergo various reaction steps to produce syngas, mainly comprising H2, CO, CO2 and CH4. H2 yield can be enhanced by means of the water–gas shift and steam reforming reactions.19,20 Key process stages during biomass gasification for syngas production are indicated by following reactions:
| Biomass → gas + tars + char | (1) |
| Tars → light and heavy hydrocarbons + H2 + CO2 + CO + CH4 | (2) |
| Char → H2 + CO2 + CO + CH4 + ash/char (solid residual) | (3) |
The formation mechanisms of H2, CO2, CO, CH4 and water (steam) during gasification are facilitated by the following reactions [eqn (4)–(10)] occurring under different mass kinetics conditions.
| C + 1/2O2 ↔ CO carbon–oxygen reaction | (4) |
| C + H2O ↔ H2 + CO carbon–water (steam) reaction | (5) |
| C + CO2 ↔ 2CO Boudouard reaction | (6) |
| C + 2H2 ↔ CH4 hydrogenation reaction | (7) |
| CO + H2O ↔ H2 + CO2 water–gas shift reaction | (8) |
| CO + 3H2 ↔ CH4 + H2O methanation reaction | (9) |
| CH4 + H2O ↔ CO + 3H2 steam–methane reforming reaction | (10) |
However, the yield and composition of gaseous products resulting from biomass gasification are highly dependent on several factors such as the feedstock type, composition, reactor temperature, biomass feeding rate, equivalence ratio (ER) and type of reactor.17
The commercial exploitation of microalgal biomass for cost effective biofuel production is hindered by the challenges of high investment costs and energy input required for microalgae cultivation and biomass harvesting.20,21 To develop microalgae as future sustainable fuel resources, research investigations into different aspects of microalgae-to-biofuel upstream and downstream processes have been reported, and these usually cover the optimization of biomass productivity during cultivation14,20,22 and evaluation of the process parameters affecting microalgal biofuel production.23–26 Though such research efforts have somewhat resulted in process improvements in the biochemical development of biodiesel and bioethanol from microalgal biomass, only a few studies have been reported on the gasification of microalgal biomass to produce syngas products. This has certainly retarded progress in the achievement of commercial scale production of syngas from microalgal biomass. Hirano et al.27 gasified a Spirulina sp. biomass slurry at three different temperatures; 850 °C, 950 °C and 1000 °C at a continuous feed rate of 0.25 g min−1. The product gas mainly comprised H2, CO, CO2 and CH4 with minor concentrations of O2, N2 and C2H4. H2 concentration increased with increasing temperature, whereas CO2, CO and CH4 concentration decreased with increasing temperature. They also reported an increase in carbon conversion from 93% to 100% after elevating the reaction temperature from 850 °C to 1000 °C. Minowa et al.28 investigated the gasification of C. vulgaris biomass at a temperature of 350 °C under a nitrogen cycling system in the presence of a Ni catalyst. The catalytic gasification system rather favoured the production of CH4 and not H2. The total gas yield and carbon conversion increased with increasing catalyst loading. The gasification process resulted in the conversion of the nitrogen present in the biomass to ammonia. Moreover, Stucki et al.29 reported the catalytic gasification of S. platensis at 400 °C using a ruthenium catalyst. They reported that increasing the catalyst loading increases carbon gasification up to 50% and the methane concentration up to 40%. Also increasing the catalyst to microalgal biomass ratio increases the CH4/H2 ratio. Chakinala et al.30 studied both the catalytic and non-catalytic supercritical water gasification of C. vulgaris and reported that excessive loading of the catalyst resulted in a higher H2 production and lower CO production via a water–gas shift reaction. They concluded that higher temperatures, lower microalgae loadings and longer reaction times were suitable conditions for optimal gasification efficiency.
Most of the microalgal biomass gasification processes have relied on the use of fixed and fluidized bed-type reactors.31–33 However, major issues relating to carbon loss and scalability make these reactors less viable for commercial scale applications.34 Hence, this study employs the use of a horizontal tubular reactor for microalgal biomass gasification. Horizontal tubular reactors have successfully been applied for the gasification of different types of biomass including sawdust, grapevine prunings, grape marc and risk husks.35–37 The horizontal configuration gives significant improvements in the gasification performance by increasing the biomass particulates’ residence time, enhancing heat transfer due to a larger particle to metal surface contact, and producing less tar compared to existing vertical designs.36,37 However, the application of horizontal tubular reactors for microalgal biomass gasification with a detailed investigation of the effects of the process parameters on the syngas production yield, productivity and quality is scarcely reported. This study therefore investigates microalgal gasification for syngas production using a horizontal tubular reactor under varying process conditions of temperature, microalgal biomass loading, heating rate, and ER in order to characterize the process behavior and optimize the production of syngas.
2. Materials and methodologies
2.1. Microalgal species
The biomass C. vulgaris (green microalgae) with a high carbon content was used as the feedstock for gasification. The microalgal species was obtained from Pure Bulk Inc (USA) and delivered in a green powder form with an average particle size of 100 μm. The biomass was kept in a desiccator until further used.2.2. Characterization of microalgal biomass
Proximate analysis, ultimate analysis, and determination of the higher calorific value of C. vulgaris were carried out using a thermogravimetric analyzer (TGA) (TGA/SDTA851, Mettler Toledo, 96 USA), a CHNS/O analyzer (LECO True Spec CHNS628, USA), and a plain jacket oxygen-bomb calorimeter (Parr 1341, USA), respectively. For proximate analysis, 20 mg of fine powder placed in an alumina crucible was heated inside a furnace continuously from ambient temperature to 1000 °C at a heating rate of 10 °C min−1 under an air atmosphere at a constant flow rate of 25 mL min−1. For ultimate analysis, 1.0 mg of sample was weighed into a tin capsule and placed into the autosampler. The temperature was set at 1000 °C for the analysis. Oxygen, nitrogen and helium were used as the carrier gas. For determination of the higher calorific value, 1.0 g of sample was placed into a crucible connected to a fuse wire of 10 cm. The crucible was then fixed inside the steel pressure vessel of the colorimeter, known as the steel bomb. The bomb was closed and charged with oxygen at 30 bars to ensure complete combustion of the sample. The system was placed in a calorimeter bucket filled with 2000 grams of tap water, and the unit was stirred for 5 min until thermal equilibrium was established. Ignition was started and the heat of combustion was determined at 30 s intervals throughout the combustion process. The measured data were used to determine the calorific value. The results of the proximate analysis and ultimate analysis, and the higher calorific value of the C. vulgaris biomass are given in Table 1.| Biomass characterization | Composition (wt%) |
|---|---|
| a The oxygen was determined by difference (100% − C − H − N − S = O). | |
| Proximate analysis | |
| Moisture | 6.3 ± 0.3 |
| Volatile matter | 83.5 ± 0.1 |
| Fixed carbon | 3.8 ± 0.4 |
| Ash | 5.1 ± 0.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Ultimate analysis | |
| C | 50.39 ± 1.6 |
| H | 6.01 ± 0.7 |
| N | 14.77 ± 3.3 |
| S | 6.05 ± 0.5 |
| Oa | 22.78 ± 2.0 |
| HCV (MJ kg−1) | 22.5 |
2.3. Equipment facility
Fig. 1 shows a schematic diagram of the experimental setup used for gasification, consisting of a tubular reactor, gasification software, a computer and a furnace. The unit is equipped with (1) a modulating temperature control, (2) an air flow meter, (3) an air tank, (4) a flanged inlet of air flow, (5) a safety valve, (6) a quartz sampling boat, (7) a horizontal quartz tubing reactor, (8) an electric furnace, (9) a flanged outlet air flow, and (10) a gas sampling bag. The quartz tube reactor is placed horizontally inside an electric furnace with a diameter and length of 15 mm and 15.6 inch, respectively. The furnace was operated at atmospheric pressure under isothermal conditions. The reactor was fed with biomass using the sampling quartz boat. The gasification conditions were input through the computer to activate the furnace operation at the set conditions. The reactor temperature conditions were divided into three stages: (i) initial temperature stabilization at 50 °C for 5 min, (ii) attainment of the desired thermal equilibrium temperature at the desired heating rate and (iii) reaction stabilization for 20 min before completion. A type k thermocouple connected to the temperature controller read the reaction temperature during gasification to enable temperature control. Air was sparged through the reactor to act as a gasifying agent. The reactor was purged with N2 at a flow rate of 10 mL min−1 for 5 min at the end of each experiment to remove entrapped gas molecules. The gaseous products from the reactor were passed through a moisture/tar trap of 8′′ in length filled with molecular sieves and silica gel to trap solid particles and moisture. The solid/moisture-free gas product was collected into a 3 L (9′′ × 11.5′′) Tedlar gas sampling bag. After each experiment, the reactor was cooled down to ambient temperature, and byproducts such as residual liquid, char, and char samples were removed. The byproducts were weighed and stored in glass bottles for analysis.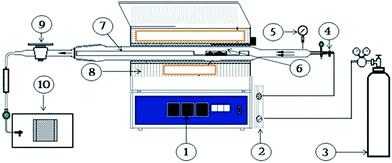 | ||
| Fig. 1 A schematic diagram of the high temperature horizontal tubular furnace unit for biomass gasification. | ||
2.4. Analysis of gaseous products
The compositions of gaseous products such as H2, CO, CO2, and CH4 were analyzed using a gas chromatographic system (Agilent 6890N; G 1540N) equipped with both Varian capillary columns (HP-PLOT/Q) and molecular sieve columns (HP-MOLSIV), and a thermal conductivity detector (TCD). Helium was used as a carrier gas. 0.25 mL of the gaseous product sample was manually injected into the column at 60 °C. Initially, stripping of CO2 from the other gaseous products occurred in the HP-PLOT/Q column and the following fractionation of H2, CH4 and CO occurred in the HP-MOLSIV column via a synchronized dual-valve injection system. The total gas yield (wt%) was estimated by calculating the difference between the weight of microalgal biomass fed into the reactor and the weights of the tar and char products. This method of total gas yield determination has been previously reported and used in several studies.38–402.5. Equivalence ratio (ER)
The ER or stoichiometric air ratio has been frequently used to identify various “oxidation regimes” during biomass conversion by gasification. It is a ratio of the actual airflow rate to the stoichiometric airflow rate required to complete the biomass combustion process under partial oxidation. ER can be expressed as follows:where m denotes mass.
ER > 1 represents a combustion process, ER = 0 indicates pyrolysis and 0 < ER < 1 represents gasification. ER is an important parameter that affects the yield and composition of gasification products. In this study, the ER was calculated on the basis of the known air flow rate (mL−1) and biomass quantity (g). An ER range of 0.2–0.35 was used to investigate its effect on microalgal biomass gasification.
2.6. Experimental design
A central composite design (CCD) approach was used to optimize the gasification process parameters to enhance syngas production. Temperature, microalgal biomass loading, heating rate and ER were the process parameters considered for the CCD analysis. The parameters were investigated at five different levels within the following ranges: temperature (500–900 °C), microalgal biomass loading (0.6–2.5 g), heating rate (5–25 °C min−1) and equivalence ratio (ER = 0.1–0.35). Table 2 shows the 5 levels of experimental design for the process parameters under investigation. Temperature, microalgae loading, heating rate and equivalence ratio have been determined as important parameters influencing syngas production.41–45| Process parameters | Symbol | Levels | ||||
|---|---|---|---|---|---|---|
| Coded | −2 | −1 | 0 | +1 | +2 | |
| Temperature (°C) | A | 500 | 600 | 700 | 800 | 900 |
| Microalgal biomass loading (g) | B | 0.6 | 1.0 | 1.5 | 2.0 | 2.5 |
| Heating rate (°C min−1) | C | 5 | 10 | 15 | 20 | 25 |
| ER | D | 0.10 | 0.20 | 0.26 | 0.30 | 0.35 |
2.7. Response variables
The quantity of syngas produced from a unit of microalgal biomass is important during gasification, and this is a key response variable. More specifically, for syngas production, the most important response variables are H2 and CO. However, the investigated response variables for this study were H2, CO, CO2, and CH4 (expressed in mol%). CO2 and CH4 were included as they are potential products of biomass gasification reactions as shown by the reaction schemes in the introduction section. All four constituents were detected by GC and the total gas composition was 100% normalized. Table 3 shows the experimental design and results for the C. vulgaris biomass gasification. The results from the experimental work were statistically analyzed using Statistica Software (StatSoft, v.10.0) in order to identify the significant parameters that affect syngas production from the microalgal biomass. Based on the data, the software analyzes the results and produces model equations for determining syngas yields. These yields represent syngas production based on the four parameters investigated. The equations will be used to predict the syngas yields and compared with the yields generated experimentally. The software analyzes the experimental data based on the influence of each process variable and possible synergies to generate a polynomial function which quantifies the effect of the process variables on syngas production and the conditions for optimal syngas production. This optimization approach and methodology is widely reported.46–48| Temperature (A) | Microalgal biomass loading (B) | Heating rate (C) | ER (D) | Response variables (mol%) | Product distribution (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | Tar | Char | Gas | |||||
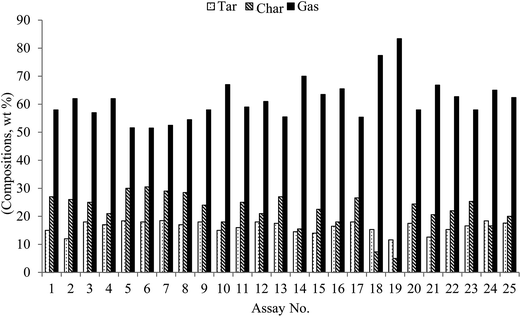
| 1 | (−1) | (−1) | (−1) | (−1) | 13.42 | 21.00 | 44.00 | 21.51 | 15.0 | 27.0 | 58.0 |
| 2 | (−1) | (−1) | (−1) | (1) | 12.43 | 24.50 | 42.34 | 20.70 | 12.0 | 26.0 | 62.0 |
| 3 | (−1) | (−1) | (1) | (−1) | 19.75 | 23.16 | 37.58 | 19.49 | 18.0 | 25.0 | 57.0 |
| 4 | (−1) | (−1) | (1) | (1) | 19.42 | 25.62 | 37.78 | 17.16 | 17.0 | 21.0 | 62.0 |
| 5 | (−1) | (1) | (−1) | (−1) | 22.31 | 24.41 | 36.96 | 16.29 | 18.4 | 30.0 | 51.6 |
| 6 | (−1) | (1) | (−1) | (1) | 14.65 | 26.00 | 39.82 | 19.46 | 18.0 | 30.5 | 51.5 |
| 7 | (−1) | (1) | (1) | (−1) | 26.63 | 19.21 | 36.85 | 17.30 | 18.5 | 29.0 | 52.5 |
| 8 | (−1) | (1) | (1) | (1) | 19.97 | 20.29 | 37.93 | 21.79 | 17.0 | 28.5 | 54.5 |
| 9 | (1) | (−1) | (−1) | (−1) | 22.38 | 28.36 | 32.47 | 16.77 | 18.0 | 24.0 | 58.0 |
| 10 | (1) | (−1) | (−1) | (1) | 23.76 | 30.37 | 36.16 | 09.69 | 15.0 | 18.0 | 67.0 |
| 11 | (1) | (−1) | (1) | (−1) | 19.98 | 35.39 | 27.84 | 16.76 | 16.0 | 25.0 | 59.0 |
| 12 | (1) | (−1) | (1) | (1) | 20.76 | 37.35 | 30.10 | 11.77 | 18.0 | 21.0 | 61.0 |
| 13 | (1) | (1) | (−1) | (−1) | 31.40 | 26.86 | 28.33 | 13.33 | 17.5 | 27.0 | 55.5 |
| 14 | (1) | (1) | (−1) | (1) | 26.44 | 27.15 | 33.25 | 13.14 | 14.5 | 15.5 | 70.0 |
| 15 | (1) | (1) | (1) | (−1) | 24.73 | 29.64 | 27.12 | 18.49 | 14.0 | 22.5 | 63.5 |
| 16 | (1) | (1) | (1) | (1) | 22.19 | 28.00 | 30.77 | 19.00 | 16.5 | 18.0 | 65.5 |
| 17 | (−2) | (0) | (0) | (0) | 14.29 | 26.45 | 44.62 | 14.62 | 18.0 | 26.6 | 55.4 |
| 18 | (2) | (0) | (0) | (0) | 28.15 | 40.81 | 20.41 | 10.61 | 15.3 | 07.3 | 77.4 |
| 19 | (0) | (−2) | (0) | (0) | 18.16 | 26.00 | 42.45 | 13.24 | 11.6 | 5.00 | 83.4 |
| 20 | (0) | (2) | (0) | (0) | 25.16 | 23.41 | 35.46 | 15.95 | 17.5 | 24.4 | 58.0 |
| 21 | (0) | (0) | (−2) | (0) | 17.20 | 22.73 | 42.56 | 17.46 | 12.6 | 20.6 | 66.8 |
| 22 | (0) | (0) | (2) | (0) | 20.90 | 24.48 | 32.16 | 22.41 | 15.3 | 22.0 | 62.7 |
| 23 | (0) | (0) | (0) | (−2) | 27.54 | 21.32 | 26.75 | 25.36 | 16.6 | 25.3 | 58.0 |
| 24 | (0) | (0) | (0) | (2) | 19.69 | 22.30 | 36.65 | 21.34 | 18.4 | 16.6 | 65.0 |
| 25 | (0) | (0) | (0) | (0) | 19.87 | 20.46 | 42.35 | 17.80 | 17.6 | 20.0 | 62.4 |
3. Results and discussion
3.1. Analysis of response variables
Table 3 shows the experimental design and the product distribution results for the C. vulgaris biomass gasification. CO2 was determined as the dominant gas produced during the gasification process (44.62 mol%). This yield was obtained during biomass gasification at 500 °C with 1.5 g of solid biomass loading, a heating rate of 15 °C min−1, and an ER of 0.26. The lowest CO2 yield of 20.41 mol% was obtained when a higher temperature of 900 °C was applied whilst the other process parameters remained the same as for the highest yield.The maximum CO production yield of 40.81 mol% was achieved during gasification at 900 °C with 1.5 g microalgae loading, a 15 °C min−1 heating rate, and an ER of 0.26. The lowest CO yield of 19.21 mol% was obtained during gasification at 600 °C with 2.0 g microalgae loading, a 20 °C min−1 heating rate, and an ER of 0.2.
Also, the maximum H2 production yield of 31.40 mol% was obtained during gasification at 800 °C with 2.0 g microalgae loading, a 10 °C min−1 heating rate, and an ER of 0.2. However, the yield of H2 dropped to 12.43 mol% during gasification at 600 °C with 1.0 g microalgae loading, a 10 °C min−1 heating rate, and an ER of 0.3.
With the variations in product yields resulting from changes in the process parameters, it can be established that temperature, amount of microalgae loaded, heating rate, and ER are important parameters that affect the quantity and composition of gasification product yields.
Fig. 2 shows the response models from the experimental data in three dimensional surface plots for the response variables, H2, CO, CO2, and CH4. The images (a–h) in Fig. 2 represent the response plots generated from changes in the process parameters.
It was generally observed that the H2 yield (Fig. 2a and b) continuously increased with increasing reactor temperature, microalgal biomass loading and heating rate. The positive effect of increasing the microalgae loading can be attributed to the increase in the mass of reactive species per unit volume to shift the equilibrium of the water–gas shift reaction (CO + H2O ↔ H2 + CO2) to the right. However, the H2 yield (Fig. 2b) decreased by increasing the ER within the given temperature range (Table 3). Injecting excess oxygen into the gasification system (increasing the ER) for shorter contact durations resulted in negative effects on the H2 (Fig. 2b) and CH4 (Fig. 2h) production yields. Under these conditions, the rate of the oxidation reaction (C + O2↔ CO2) is enhanced, resulting in an elevated CO2 production.
CO yields were also significantly influenced by increasing the reactor temperature, ER and heating rate as shown in Fig. 2c and d. The overall results showed that higher microalgal biomass loading and ER at lower temperatures do not favor H2 and CO production.
The elevated H2 and CO yields observed after increasing the gasification temperature were facilitated by the endothermic nature of the primary gasification reaction of C + H2O ↔ CO + H2 and the secondary reaction of C + 2H2O ↔ CO2 + 2H2 at a lower heating rate (10 °C min−1) and air ratio (ER = 0.2).
Likewise, the occurrence of steam, generated mainly from moisture present in the biomass and the reactive gas, in a reactive atmosphere favors H2 production. Supramono et al.49 observed a H2 yield increment of 30–32 mol% and a CO yield increment of 25–35 mol% by increasing the reactor temperature from 750 °C to 850 °C for steam gasification of lignite char. Chen et al.50 also reported similar increments for H2 (13–42 mol%) and CO (22–32 mol%) by increasing the reactor temperature from 600 to 800 °C for steam gasification of an acid-hydrolyzed residue of corncob. The presence of steam/moisture resulting from the increase in temperature increases the H2 and CO yields, due to the fact that syngas (H2 + CO) production is mainly dependent on interactions between temperature and steam supported by steam gasification reactions, which are tremendously endothermic.
The observed increases in H2 and CO yields resulting from the reactor temperature elevation, and a H2 yield increment resulting from an increased biomass loading have previously been observed in other studies.47,50–52
The trend reported in Table 3 shows that the effect of temperature on the H2 and CO yields varies over the range of temperatures investigated. The H2 yield (Fig. 2a) and CO yield (Fig. 2c) were observed to increase from 14.29 to 31.40 mol% for 500–800 °C, and 26.45 to 40.81 mol% for 500–900 °C temperature increases. However, the H2 yield did not show any increasing pattern at 900 °C, and this could be due to the minimal impact of the water–gas shift reaction (CO + H2O ↔ H2 + CO2) at high temperatures post-equilibrium since the amount of CO at 900 °C was significantly higher compared to that at lower temperatures. Fig. 2e and f show the temperature as a highly significant process parameter; CO2 concentration decreased considerably with increasing reactor temperature. The CO2 yield at a lower temperature (500 °C, 44.62 mol%) was significantly higher compared to that at a higher temperature (20.41 mol% at 900 °C), about 50% of a reduction. The CO2 yield increased by increasing the ER, as it enabled complete combustion in the presence of excess air. The results were also in agreement with the work reported by Rupesh et al.53 Guo et al.42 also reported that the CO2 yield increased with an increasing ER from 0.22 to 0.37. A similar effect of temperature was observed for CH4 production (Fig. 2g and h).
Though the effect of temperature on the CH4 yield varied, a decrease in the CH4 yield of ≈50% (16.46 mol% to 8.83 mol%) and an increase in the CO yield of ≈50% (26.45 mol% to 40.81 mol%) were observed after increasing the reaction temperature from 500 °C to 900 °C. This is ascribed to the domination of the steam and dry methane reforming reactions (CH4 + H2O ↔ CO + 3H2 and CH4 + CO2 ↔ 2H2 + CO) which are thermodynamically favored at higher reaction temperatures.
3.2. Statistical analysis
The response results from the CCD were incorporated into a regression model and analyzed for individual model coefficients and fitness. The data were statistically analyzed to identify the significant process parameters affecting syngas production from microalgal biomass. Analysis of variance (ANOVA) was performed to verify the model significance by Statistica (Statsoft v. 10.0), and the results are shown in Table 4. ANOVA analysis can be used to determine the model significance from the p-value at a 95% confidence level. The p-value represents the probability of obtaining results closer to the actual experimental values; thus, a smaller p-value indicates that the resultant coefficient is significant.41 The small p-value (<0.05) obtained from the ANOVA analysis demonstrates that the experimental results and the predicted values are in good agreement, as presented in Fig. 5.| Response variables | H2 (mol%) | CO (mol%) | CO2 (mol%) | CH4 (mol%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | DF | P | SS | DF | P | SS | DF | P | SS | DF | P | |
| a SS: sum of squares; DF: degree of freedom. | ||||||||||||
| A | 201 | 1 | 0.00 | 322.40 | 1 | 0.00 | 563.10 | 1 | 0.00 | 70.56 | 1 | 0.00 |
| A × A | 1.02 | 1 | 0.22 | 141.43 | 1 | 0.00 | 70.50 | 1 | 0.00 | 22.91 | 1 | 0.00 |
| B | 111.04 | 1 | 0.00 | 39.55 | 1 | 0.00 | 37.29 | 1 | 0.01 | 3.73 | 1 | 0.14 |
| B × B | 1.48 | 1 | 0.14 | 19.77 | 1 | 0.00 | 8.34 | 1 | 0.17 | 9.70 | 1 | 0.02 |
| C | 7.70 | 1 | 0.00 | 7.97 | 1 | 0.00 | 94.61 | 1 | 0.00 | 17.32 | 1 | 0.00 |
| C × C | 0.75 | 1 | 0.28 | 10.31 | 1 | 0.00 | 17.64 | 1 | 0.06 | 2.40 | 1 | 0.23 |
| D | 36.27 | 1 | 0.00 | 8.78 | 1 | 0.00 | 17.41 | 1 | 0.06 | 1.98 | 1 | 0.27 |
| D × D | 5.05 | 1 | 0.01 | 3.73 | 1 | 0.05 | 66.42 | 1 | 0.00 | 17.54 | 1 | 0.00 |
| A × B | 0.02 | 1 | 0.83 | 14.91 | 1 | 0.00 | 0.57 | 1 | 0.70 | 10.54 | 1 | 0.02 |
| A × C | 96.43 | 1 | 0.00 | 39.91 | 1 | 0.00 | 0.12 | 1 | 0.86 | 14.64 | 1 | 0.01 |
| A × D | 7.63 | 1 | 0.00 | 2.30 | 1 | 0.11 | 6.24 | 1 | 0.23 | 13.91 | 1 | 0.01 |
| B × C | 5.29 | 1 | 0.01 | 37.73 | 1 | 0.00 | 15.96 | 1 | 0.07 | 19.91 | 1 | 0.00 |
| B × D | 33.13 | 1 | 0.00 | 4.03 | 1 | 0.04 | 3.44 | 1 | 0.37 | 34.91 | 1 | 0.00 |
| C × D | 0.99 | 1 | 0.22 | 0.85 | 1 | 0.32 | 0.78 | 1 | 0.66 | 0.62 | 1 | 0.53 |
| Lack of fit | 5.96 | 10 | 7.91 | 10 | 39.35 | 10 | 15.10 | 10 | ||||
| Total | 553.66 | 24 | 682.09 | 24 | 947.67 | 24 | 364.25 | 24 | ||||
| R2 | 0.98 | 0.98 | 0.95 | 0.96 | ||||||||
| Adj-R2 | 0.97 | 0.97 | 0.90 | 0.90 | ||||||||
Also, the accuracy of the model towards actual data was further analyzed by the regression coefficient (R2) and adjusted regression coefficient (Adj-R2). The range for the regression coefficient is within the range of 0–1, and a value that is close to unity demonstrates a good approximation of the model to the output response. The obtained regression coefficient and adjusted regression coefficient values for the response variables H2, CO, CO2, and CH4 were R2 = 0.98 and Adj.-R2 = 0.97, R2 = 0.98 and Adj.-R2 = 0.97, R2 = 0.95 and Adj.-R2 = 0.90, and R2 = 0.96 and Adj.-R2 = 0.90 respectively. In addition, if the value of R2 upsurges as the number of process parameters in the model increases, it is more important to use Adj-R2 which gives more accurate results. Adj-R2 shows a decreasing trend with the inclusion of irrelevant variables to the model.47
ANOVA analysis also evaluates the synergistic interactions of two process parameters towards an output response. From the statistical analysis, the syngas yield (mol%) for an individual constituent can be calculated from the equations in Table 5.
| Response variables (mol%) | Polynomial model equations | Optimum conditions |
|---|---|---|
| a The interactions between the process parameters have been taken into consideration to enable a comprehensive understanding of the effects of individual and combined process parameters on the response variables. +ve or −ve signs show whether the particular algebraic term contributes a positive or negative impact on the syngas production. | ||
| H2 | 18.35321A + 13.64196B − 12.7125AC − 7.79677D − 7.45158BD + 3.593331C + 3.576821AD − 2.97747BC + 2.91141D2 + 1.574525B2 + 1.307953A2 + 1.293725CD − 1.12474C2 − 0.213601AB | A = 705.10 °C, B = 1.44 g, C = 22.24 °C min−1, D = 0.29 |
| CO | 20.17842A + 13.36472A2 + 7.099585AC − 7.06806B − 6.90292BC + 4.996931B2 − 4.34066AB + 3.608552C2 + 3.330508D + 3.173145C − 2.25673BD + 2.172222D2 − 1.70508AD − 1.04121CD | A = 637.6 °C, B = 1.62 g, C = 16.52 °C min−1, D = 0.2 |
| CO2 | −11.9612A − 4.90287C − 4.2323A2 − 4.10807D2 − 3.07813B − 2.11744C2 + 2.103615D + 2.013715BC − 1.45579B2 + 1.260008AD + 0.9353584BD − 0.44682CD + 0.3830848AB − 0.176421AC | A = 595 °C, B = 0.69 g, C = 7.8 °C min−1, D = 0.24 |
| CH4 | −6.83413A + 4.807288BD − 3.89487A2 + 3.630552BC + 3.407684D2 + 3.385933C + 3.113935AC − 3.03533AD + 2.642065AB − 2.53447B2 + 1.571542B + 1.262309C2 − 1.14735D + 0.6435957CD | A = 634 °C, B = 1.42 g, C = 14.74 °C min−1, D = 0.24 |
3.3. Composition of reaction products
Table 3 and Fig. 3 show the product distributions for the gasification reactions of microalgal biomass for various process parameters. The product mass generation from each run was >90%. Gaseous products were found to be dominant under low or high temperature conditions. Conversely, char was predominant for low ER or heating rate conditions. The production rate of tar and char decreased under high ER, low heating rate and high temperature gasification process conditions, indicating that the temperature and ER play significant roles in the biomass decomposition and gas formation reactions. For instance, the mass of gaseous products generally decreased considerably with increasing heating rate and microalgal biomass loading under low ER and low temperature conditions.Though the composition of tar and char were high in the current study, the content of tar was relatively low compared to that obtained using hydrothermal liquefaction.54–56 Anastasakis et al.57 reported a 19.3 wt% maximum tar yield after the gasification of Saccharina (brown algae), whereas a maximum tar yield of 18.5 wt% was achieved in the present study. The difference in tar yield is possibly due to the higher temperature of gasification in the present study. The formation of tar is undesirable during biomass gasification as it contributes additional downstream requirements to enhance the productivity, conversion rate, recovery and quality of the gasification process. A compositional study of tar shows that it contains a wide range of aromatic compounds and their derivatives including alkyl benzenes and phenols.58 Some of these aromatic compounds are often complex and resist decomposition during gasification.58 However, these compounds together with light aliphatic hydrocarbons including CH4 can undergo catalytic cracking or reforming reactions to enhance gaseous product yields under specific temperature conditions.59–62
3.4. Process optimization to achieve the maximum H2 yield
The optimized process parameters and the conditions under which they were obtained are shown in Table 5. The yield of H2 obtained is compared with previously reported yields in Table 6. The optimum process parameters as detailed in Table 5 were a temperature of 703 °C, a microalgal biomass loading of 1.44 g, a heating rate of 22.24 °C min−1, and an ER of 2.9. The optimum H2 yield was 41.75 mol%, which is >30% more than the maximum yield obtained from the experimental design. The reproducibility of the optimum yield of H2 was verified experimentally with an acceptable accuracy as shown in Fig. 4. Table 6 shows that the produced syngas has desirable compositions in terms of higher H2 and lower CO2 yields compared to other reported microalgal biomass gasification studies. The key process advantage of the current technology is the production of a high syngas yield under low temperature conditions using air as the gasification agent. Other reported processes mostly use steam, catalysts and high temperatures, making the whole process lengthy, costly (steam is costly compared to air and using a catalyst requires a two-way process) and environmentally unsustainable. The gasification method developed has the advantages of a reduced process time and a better economic outlook.| Experimental conditions | Feedstock type | Yield (mol%) | Reference |
|---|---|---|---|
| a A: temperature; B: microalgal biomass loading; C: heating rate; D: ER, X: gasifying agent. | |||
| A = 705.10 °C, B = 1.44 g, C = 22.24 °C min−1, D = 0.29, X = air | C. vulgaris | H2 = 41.75, CO = 18.63, CO2 = 24.40, CH4 = 15.19 | Present work |
| A = 500 °C, B = 1.0 g, C = 30 °C min−1, MPa = 36, X = steam | Spirulina | H2 = 21.1, CO = 4.26, CO2 = 36.2, CH4 = 21.2 | Onwudili et al.58 |
| Saccharina | H2 = 24, CO = 4.23, CO2 = 50.2, CH4 = 12 | Onwudili et al.58 | |
| Chlorella | H2 = 18.3, CO = 5.28, CO2 = 45, CH4 = 17 | Onwudili et al.58 | |
| A = 800 °C, D = 0.4, X = air/steam | Spirulina platensis | H2 = 19, CO = 40, CO2 = 25, CH4 = 8 | Yang et al.63 |
| A = 825 °C, B = 2, C = 3 °C min−1, X = air/steam | Lignite coal + K2CO3 | H2 = 32, CO = 35, CO2 = 9, CH4 = 2 | Supramono et al.49 |
3.5. Validation of model
The accuracy of the model was verified by analyzing the predicted and actual experimental results. As shown in Fig. 5, the straight lines demonstrate the agreement between predicted and actual experimental values, representing an acceptable correlation between the process conditions (temperature, microalgal biomass loading, heating rate and ER) and the response variables (H2, CO, CO2, and CH4) for the experimental conditions of C. vulgaris biomass gasification.4. Conclusion
CCD was used to optimize the process parameters of C. vulgaris biomass gasification for high syngas production. The effects of the gasification process parameters (temperature, microalgal biomass loading, heating rate and ER) on the response variables (H2, CO, CO2, and CH4) were investigated. The process parameters and their synergistic effects provide useful information to maximize syngas yield. Amongst the investigated process parameters, temperature and microalgal biomass loading were the most influential parameters in increasing the H2 yield. The optimum yield of H2 was obtained at 703 °C with a microalgal biomass loading of 1.44 g, a heating rate of 22.24 °C min−1, and an ER value of 0.29. Significant increments in the yields of H2 and CO were observed with increasing temperature. An increase in the ER during the gasification process resulted in decreasing H2 and CH4 yields. A higher heating rate was observed to favour H2, CO and CH4 production. Statistical analysis showed sufficient agreement between the experimental and predicted results. Comparing the findings with other reported studies, it is anticipated that the use of a horizontal tubular reactor for the direct gasification of microalgal biomass with further process characterization and optimization will represent a significant step in escalating interest in process design for commercial scale applications.References
- S. J. Yoon, Y. C. Choi and J. G. Lee, Energy Convers. Manage., 2010, 51, 42–47 CrossRef CAS PubMed.
- P. Lv, Z. Yuan, C. Wu, L. Ma, Y. Chen and N. Tsubaki, Energy Convers. Manage., 2007, 48, 1132–1139 CrossRef CAS PubMed.
- M. R. Mahishi, M. S. Sardrameli, S. Vigavaraghavan and D. Y. Goswami, J. Eng. Gas Turbines Power, 2008, 130, 1–8 CrossRef.
- Y. H. Taufiq-Yap, S. Sivasangar and A. Salmiaton, Energy, 2012, 47, 158–165 CrossRef CAS PubMed.
- M. J. Antal, S. G. Allen, D. Schulman and X. Xu, Ind. Eng. Chem. Res., 2000, 39, 4040–4053 CrossRef CAS.
- A. V. Ensinas, S. A. Nebra, M. A. Lozano and L. M. Serra, Energy Convers. Manage., 2007, 48, 2978–2987 CrossRef CAS PubMed.
- M. Balat, Int. J. Green Energy, 2008, 5, 212–238 CrossRef CAS PubMed.
- S. Caro, D. Torres and M. Toledo, Int. J. Hydrogen Energy, 2015, 6, 2568–2577 CrossRef PubMed.
- P. S. Nigam and A. Singh, Prog. Energy Combust. Sci., 2011, 37, 52–68 CrossRef CAS PubMed.
- B. Maddi, S. Viamajala and S. Varanasi, Bioresour. Technol., 2011, 102, 11018–11026 CrossRef CAS PubMed.
- P. McKendry, Bioresour. Technol., 2002, 83, 37–46 CrossRef CAS.
- W. H. Chen, Z. Y. Wu and J. S. Chang, Bioresour. Technol., 2014, 155, 245–251 CrossRef CAS PubMed.
- X. Miao, Q. Wu and C. Yang, Fast pyrolysis of microalgae to product renewable fuels, J. Anal. Appl. Pyrolysis, 2004, 71, 855–863 CrossRef CAS PubMed.
- A. F. Clarens, E. P. Resurreccion, M. A. White and L. M. Colosi, Environ. Sci. Technol., 2010, 44, 1813–1819 CrossRef CAS PubMed.
- V. Skorupskaite, V. Makareviciene and D. Levisauskas, Algal Res., 2015, 7, 45–50 CrossRef PubMed.
- A. Kumar, D. D. Jones and M. A. Hanna, Energies, 2009, 2, 556–581 CrossRef CAS PubMed.
- I. I. Ahmed and A. K. Gupta, Appl. Energy, 2012, 91, 75–81 CrossRef CAS PubMed.
- A. Raheem, W. A. K. G. Wan Azlina, Y. H. Taufiq Yap and M. K. Danquah, Renewable Sustainable Energy Rev., 2015, 49, 990–999 CrossRef CAS PubMed.
- P. McKendry, Bioresour. Technol., 2003, 83, 47–54 CrossRef.
- L. Brennan and P. Owemde, Renewable Sustainable Energy Rev., 2010, 14, 557–577 CrossRef CAS PubMed.
- S. Ho, H. X. Ye, T. Hasunuma, J. S. Chang and A. Kondo, Perspectives on engineering strategies for improving biofuel production from microalgae—a critical review, Biotechnol. Adv., 2014, 32, 1448–1459 CrossRef CAS PubMed.
- M. Azma, M. S. Mohamed, R. Mohamad, R. A. Rahim and A. B. Ariff, Biochem. Eng. J., 2011, 53, 187–195 CrossRef CAS PubMed.
- V. Makareviciene, V. Skorupskaite, D. Levisauskas, V. Andruleviciute and K. Kazancev, Int. J. Green Energy, 2014, 11, 527–541 CrossRef CAS PubMed.
- P. D. Patil, V. G. Gude, A. Mannarswamy, S. Deng, P. Cooke, S. Munson-McGee, I. Rhodes, P. Lammers and N. Nirmalakhandan, Bioresour. Technol., 2011, 102, 118–122 CrossRef CAS PubMed.
- E. A. Ehimen, Z. F. Sun, C. G. Carrington, E. J. Birch and J. J. Eaton-Rye, Appl. Energy, 2011, 88, 3454–3463 CrossRef CAS PubMed.
- Y. Nan, J. Liu, R. Lin and L. L. Tavlarides, J. Supercrit. Fluids, 2015, 97, 174–182 CrossRef CAS PubMed.
- A. Hirano, K. Hon-Nami, S. Kunito, M. Hada and Y. Ogushi, Catal. Today, 1998, 45, 399–404 CrossRef CAS.
- T. Minowa, S. Y. Yokoyama, M. Kishimoto and T. Okakura, Fuel, 1995, 74, 1735–1738 CrossRef CAS.
- S. Stucki, F. Vogel, C. Ludwig, A. G. Haiduc and M. Brandenberger, Energy Environ. Sci., 2009, 2, 535–541 CAS.
- A. G. Chakinala, D. W. F. Brilman, W. P. M. V. Swaaij and S. R. A. Kersten, Ind. Eng. Chem. Res., 2010, 49, 1113–1122 CrossRef CAS.
- I. K. Alghurabie, B. O. Hasan, B. Jackson, A. Kosminski and P. J. Ashman, Chem. Eng. Res. Des., 2013, 91, 1614–1624 CrossRef CAS PubMed.
- H. H. Khoo, C. Y. Koh, M. S. Shaik and P. N. Sharratt, Bioresour. Technol., 2013, 143, 298–307 CrossRef CAS PubMed.
- K. C. Yang, K. T. Wu, M. H. Hsieh, H. T. Hsu, C. S. Chen and H. W. Chen, J. Taiwan Inst. Chem. Eng., 2013, 44, 1027–1033 CrossRef CAS PubMed.
- G. S. Kumar, A. V. S. S. K. S. Gupta, M. Viswanadham and M. Abhiram, Proceedings of the 1st International Conference on Advances in Thermal and Energy Systems, 2013 Search PubMed.
- Y. J. Zhao, S. Z. Sun, H. Zhou, R. Sun, H. M. Tian, J. Y. Luan and J. A. Qian, Fuel Process. Technol., 2010, 91, 910–914 CrossRef CAS PubMed.
- Y. J. Zhao, S. Z. Sun, H. M. Tian, J. Qian, F. M. Su and F. Ling, Bioresour. Technol., 2009, 100, 6040–6044 CrossRef CAS PubMed.
- J. J. Hernandez, G. Aranda-Almansa and A. Bula, Fuel Process. Technol., 2010, 91, 681–692 CrossRef CAS PubMed.
- M. A. A. Mohammed, A. Salmiaton, W. W. Azlina, M. M. Amran and A. Fakhru’l-Razi, Energy Convers. Manage., 2011, 52, 1555–1561 CrossRef CAS PubMed.
- P. Basu, Biomass Gasification and Pyrolysis: Practical Design and Theory, Academic press, 2010, pp. 219–225 Search PubMed.
- J. Good, L. Ventress, H. Knoef, U. Zielke, P. L. Hansen, W. van de Kamp, K. Sjostrom, T. Liliedahl, Ch. Unger, J. Neeft and P. Simell, Technical Report CEN BT/TF, 2005 Search PubMed.
- Y. Nan, J. Liu, R. Lin and L. L. Tavlarides, J. Supercrit. Fluids, 2015, 97, 174–182 CrossRef CAS PubMed.
- X. J. Guo, B. Xiao, X. L. Zhang, S. Y. Luo and M. Y. He, Bioresour. Technol., 2009, 100, 1003–1006 CrossRef CAS PubMed.
- Q. Guana, P. E. Savage and C. Wei, J. Supercrit. Fluids, 2012, 61, 139–145 CrossRef PubMed.
- A. C. Freitas and R. Guirardello, Chem. Eng., 2013, 32, 553–558 Search PubMed.
- D. López-González, M. Fernandez-Lopez, J. L. Valverde and L. Sanchez-Silva, Fuel, 2014, 134, 1–10 CrossRef PubMed.
- M. V. Gil, J. Fermoso, F. Rubiera and D. Chen, Catal. Today, 2015, 242, 19–34 CrossRef CAS PubMed.
- J. Fermoso, M. V. Gil, B. Arias, M. G. Plaza, C. Pevida, J. J. Pis and F. Rubiera, Int. J. Hydrogen Energy, 2010, 35, 1191–1204 CrossRef CAS PubMed.
- S. Yusup, Z. Khan, M. M. Ahmad and N. A. Rashidi, Biomass Bioenergy, 2014, 60, 98–107 CrossRef CAS PubMed.
- D. Supramono, D. Tristantini, A. Rahayu, R. K. Suwignjo and D. H. Chendra, Procedia Chem., 2014, 9, 202–209 CrossRef CAS PubMed.
- G. Chen, J. Yao, H. Yang, B. Yan and H. Chen, Bioresour. Technol., 2015, 179, 323–330 CrossRef CAS PubMed.
- R. N. André, F. Pinto, C. Franco, M. Dias, I. Gulyurtlu, M. A. A. Matos and I. Cabrita, Fuel, 2005, 84, 1635–1644 Search PubMed.
- K. Li, R. Zhang and J. Bi, Int. J. Hydrogen Energy, 2010, 35, 2722–2726 CrossRef CAS PubMed.
- S. Rupesh, C. Muraleedharan and P. Arun, Int. Scholarly Res. Not., 2014, 1–9 CrossRef PubMed.
- Z. Shuping, W. Yulong, Y. Mingde, I. Kaleem, L. Chun and T. Tong, Energy, 2010, 35, 5406–5411 CrossRef PubMed.
- D. R. Vardon, B. K. Sharma, J. Scott, G. Yu, Z. Wang, L. Schideman, Y. Zhang and T. J. Strathmann, Bioresour. Technol., 2011, 102, 8295–8303 CrossRef CAS PubMed.
- P. Biller and A. B. Ross, Bioresour. Technol., 2011, 102, 215–225 CrossRef CAS PubMed.
- K. Anastasakis and A. B. Ross, Bioresour. Technol., 2011, 102, 4876–4883 CrossRef CAS PubMed.
- J. A. Onwudili, A. R. Lea-Langton, A. B. Ross and P. T. Williams, Bioresour. Technol., 2013, 127, 72–80 CrossRef CAS PubMed.
- C. Li and K. Suzuki, Renewable Sustainable Energy Rev., 2009, 13, 594–604 CrossRef CAS PubMed.
- L. Pérez-Moreno, J. Soler, J. Herguido and M. Menéndez, J. Power Sources, 2013, 243, 233–241 CrossRef PubMed.
- S. Zeng, X. Fu, T. Zhou, X. Wang and H. Su, Fuel Process. Technol., 2013, 114, 69–74 CrossRef CAS PubMed.
- X. Xu, P. Li and Y. Shen, Appl. Energy, 2013, 108, 202–217 CrossRef CAS PubMed.
- K. C. Yang, K. T. Wu, M. H. Hsieh, H. T. Hsu, C. S. Chen and H. W. Chen, J. Taiwan Inst. Chem. Eng., 2013, 44, 1027–1033 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |