Structure characteristics and photoactivity of simultaneous luminescence and photocatalysis in CaV2O6 nanorods synthesized by the sol–gel Pechini method
Abstract
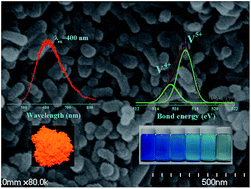
The work reports the large-scale synthesis, and simultaneous luminescent and photocatalytic activities of self-activated metavanadate CaV2O6 nanorods. The samples were synthesized using the Pechini method on the basis of a citrate-complexation route. The phase formation and crystal structure were investigated by X-ray powder diffraction (XRD) and structural refinements. The detailed surface properties were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS) and Brunauer–Emmett–Teller (BET) measurements. The photoactivity was evaluated by the photoluminescence of the as-prepared powders and the photodegradation of methylene blue (MB) solutions. CaV2O6 presents a broad emission centered at 580 nm, which is different from the luminescence in commonly reported self-activated vanadates. The results of excitation spectra and decay curves (10.5 μs) indicate only one kind of luminescence center in the lattices. In particular, it was found for the first time that CaV2O6 nanorods showed excellent photocatalytic activity. The luminescence properties and degradation mechanism were discussed based on the structure characteristics and the band structure. CaV2O6 presents a layered structure constructed by line-arranged VO5 units. The coexistence of V4+/V5+ ions and the induced oxygen vacancies in the lattices were confirmed to have a contribution to the photocatalytic activities. These results indicate that CaV2O6 could be a potential photoactive material with a two-dimensional structure.

 Please wait while we load your content...
Please wait while we load your content...