Facile fabrication of a mpg-C3N4/TiO2 heterojunction photocatalyst with enhanced visible light photoactivity toward organic pollutant degradation
Abstract
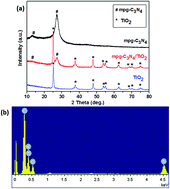
A facile hard template approach has been developed to prepare mpg-C3N4/TiO2 composites using SiO2 nanoparticles as a hard template and cyanamide as a precursor. The samples have been well characterized using X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDS), transmission electron microscopy (TEM), and ultraviolet-visible diffuse reflectance spectroscopy (UV-vis DRS). The results demonstrate that TiO2 nanoparticles sized 5–10 nm were distributed on the surface of mpg-C3N4 to form the mpg-C3N4/TiO2 heterojunction photocatalyst. In this way, the mpg-C3N4/TiO2 heterojunction catalyst has a porous structure and large surface area, which increases the contact area for pollutants. Rhodamine B (RhB) was used as a representative organic pollutant to evaluate the photocatalytic activity of the samples under visible light irradiation. The results show that the as-prepared mpg-C3N4/TiO2 heterojunction catalyst has a significantly enhanced visible light photocatalytic activity compared to pure mpg-C3N4 and that the degradation rate was 1.6 times higher than that of pure mpg-C3N4. The increased photocatalytic activity of the mpg-C3N4/TiO2 heterojunction catalyst can be attributed to the formation of the heterojunction between mpg-C3N4 and TiO2, which suppresses the recombination of photoinduced electron–hole pairs. Furthermore, based on systematic characterization and discussion, a possible photocatalytic mechanism for the excellent photocatalytic performance was proposed.

 Please wait while we load your content...
Please wait while we load your content...