Synthesis, structure and luminescence characteristics of a novel red phosphor NaLa9(GeO4)6O2:Eu3+ for light emitting diodes and field emission displays
Abstract
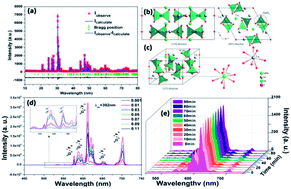
In order to explore a new kind of red phosphor for near ultraviolet white light emitting diodes (NUV-WLEDs) and field emission displays (FEDs), Eu3+ doped NaLa9(GeO4)6O2 was synthesized and its luminescence properties were studied for the first time for fundamental research. The results indicate that all samples crystallize in the hexagonal crystal system with the P63/m space group. Eu3+ doped NaLa9(GeO4)6O2 with the content of 0.07 has optimal photoluminescence properties, with a dominant red emission peak at 612 nm (5D0–7F2) with CIE coordinates of (0.64, 0.35) under 392 nm excitation and the quantum efficiency of 45.2%. Additionally, the thermal quenching property has been studied and its possible mechanism has also been expounded. Furthermore, the cathodoluminescence (CL) property was investigated and the result reveals that the sample has excellent degradation properties for FEDs. The study reveals that NaLa9(GeO4)6O2:0.07Eu3+ could be a suitable red-emitting phosphor candidate for NUV-WLEDs and FEDs.


 Please wait while we load your content...
Please wait while we load your content...